Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-02-29 , DOI: 10.2174/1570179417666191220100614 Tran Nguyen Minh An 1 , Pham Thai Phuong 1 , Nguyen Minh Quang 1, 2 , Nguyen Van Son 1 , Nguyen Van Cuong 1 , Le Van Tan 1 , Mai Dinh Tri 3, 4 , Mahboob Alam 5 , Pham Van Tat 6
|
A series of novel 1,3-thiazole derivatives (5a-i) with a modified phenothiazine moiety were synthesized and tested against cancer cell line MCF-7 for their cytotoxicity. Most of them (5a-i) were less cytotoxic or had no activity against MCF-7 cancer cell line.
Material and Methods: The IC50 value of compound (4) was 33.84 μM. The compounds (5a-i) were also evaluated for antimicrobial activities, but no significant activity was observed. The antioxidant activity was conducted for target compounds (5a-i). The IC50 value of compound (5b) was 0.151mM.
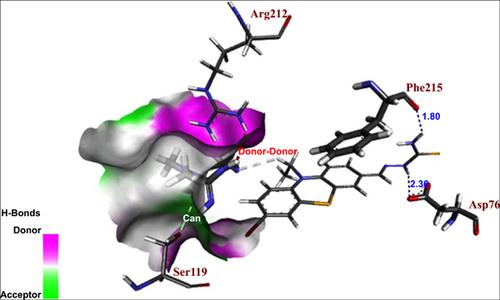
Results: The total amount of energy, ACE (atomic contact energy), energy of receptor (PDB: 5G5J), and ligand interaction of structure (4) were found to be 22.448 Kcal.mol-1 , -247.68, and -91.91 Kcal.mol-1, respectively. The structure (4) is well binded with the receptor because the values of binding energy, steric energy, and the number of hydrogen bondings are -91.91, 22.448 kcal.mol-1, and 2, respectively. It shows that structure (4) has good cytotoxicity with MCF-7 in vitro.
Conclusion: The increasing of docking ability of structures (5a-i) with the receptor is presented in increasing order as (5f)>(5e)>(5g)>(5a)>(5b)>(5d)>(5c)>(5i)>(5h). The structure bearing substitution as thiosemicarbazone (4), nitrogen heterocyclic (5f), halogen (5e), and azide (5g) showed good cytotoxicity activity in vitro.
中文翻译:

基于吩噻嗪的新型2,4-二取代噻唑的合成,对接研究,细胞毒性,抗氧化和抗菌活性。
合成了一系列具有修饰的吩噻嗪部分的新型1,3-噻唑衍生物(5a-i),并针对癌细胞系MCF-7进行了细胞毒性测试。它们中的大多数(5a-i)具有较低的细胞毒性或对MCF-7癌细胞无活性。
材料与方法:化合物(4)的IC50值为33.84μM。还评估了化合物(5a-i)的抗微生物活性,但未观察到显着的活性。对目标化合物(5a-i)进行了抗氧化活性。化合物(5b)的IC 50值为0.151mM。
结果:发现总能量,ACE(原子接触能量),受体能量(PDB:5G5J)和结构(4)的配体相互作用分别为22.448 Kcal.mol-1,-247.68和-91.91 Kcal .mol-1。结构(4)与受体结合良好,因为结合能,空间位能和氢键数分别为-91.91、22.448 kcal.mol-1和2。表明结构(4)在体外对MCF-7具有良好的细胞毒性。
结论:结构(5a-i)与受体的对接能力增加的顺序为(5f)>(5e)>(5g)>(5a)>(5b)>(5d)>(5c)。 >(5i)>(5h)。具有取代的结构,如硫代半脲(4),氮杂环(5f),卤素(5e)和叠氮化物(5g)在体外具有良好的细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号