当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The uptake of tau amyloid fibrils is facilitated by the cellular prion protein and hampers prion propagation in cultured cells.
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-05-11 , DOI: 10.1111/jnc.15040 Elena De Cecco 1 , Luigi Celauro 1 , Silvia Vanni 1 , Micaela Grandolfo 1 , Edoardo Bistaffa 2 , Fabio Moda 2 , Adriano Aguzzi 3 , Giuseppe Legname 1, 4
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-05-11 , DOI: 10.1111/jnc.15040 Elena De Cecco 1 , Luigi Celauro 1 , Silvia Vanni 1 , Micaela Grandolfo 1 , Edoardo Bistaffa 2 , Fabio Moda 2 , Adriano Aguzzi 3 , Giuseppe Legname 1, 4
Affiliation
|
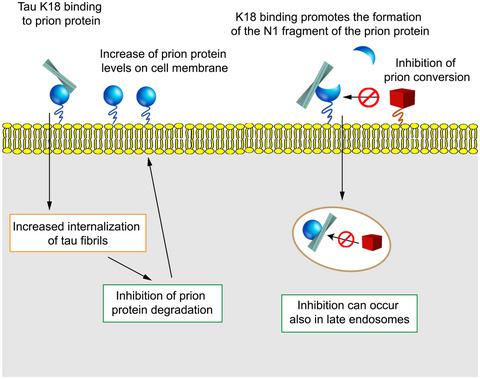
Tauopathies are prevalent, invariably fatal brain diseases for which no cure is available. Tauopathies progressively affect the brain through cell‐to‐cell transfer of tau protein amyloids, yet the spreading mechanisms remain unknown. Here we show that the cellular prion protein (PrPC) facilitates the uptake of tau aggregates by cultured cells, possibly by acting as an endocytic receptor. In mouse neuroblastoma cells, pull‐down experiments revealed that tau amyloids bind to PrPC. Confocal images of both wild‐type and PrPC ‐knockout N2a cells treated with fluorescently labeled synthetic tau fibrils showed that the internalization was reduced in isogenic cells devoid of the gene encoding PrPC. Pre‐treatment of the same cells with antibodies against N‐proximal epitopes of PrPC impaired the binding of tau amyloids and decreased their uptake. Surprisingly, exposure of chronically prion‐infected cells to tau amyloids reduced the accumulation of aggregated prion protein and this effect lasted for more than 72 hr after amyloid removal. These results point to bidirectional interactions between the two proteins: while PrPC mediates the entrance of tau fibrils in cells, PrPSc buildup is greatly reduced in their presence, possibly because of an impairment in the prion conversion process.
中文翻译:

au蛋白淀粉蛋白原纤维的摄取通过细胞病毒蛋白促进,并阻碍病毒在培养细胞中的繁殖。
关节病是普遍存在的致命性脑疾病,无法治愈。牛头颅病通过tau蛋白淀粉样蛋白的细胞间转移逐渐影响大脑,但其传播机制仍然未知。在这里,我们显示细胞病毒蛋白(PrP C)可能通过充当胞吞受体来促进培养细胞对tau聚集体的摄取。在小鼠神经母细胞瘤细胞中,下拉实验表明tau淀粉样蛋白与PrP C结合。用荧光标记的合成tau原纤维处理的野生型和PrP C敲除N2a细胞的共聚焦图像显示,在缺乏编码PrP C的基因的同基因细胞中,内化作用降低。用抗PrP C N近端表位的抗体预处理同一细胞会损害tau淀粉样蛋白的结合并降低其摄取。出乎意料的是,将长期感染ion病毒的细胞暴露于tau淀粉样蛋白会减少聚集的病毒蛋白的积累,这种作用在去除淀粉样蛋白后持续了超过72小时。这些结果表明这两种蛋白质之间存在双向相互作用:虽然PrP C介导tau纤维进入细胞,但PrP Sc的存在会大大减少其积累,这可能是由于pr病毒转化过程的损害。
更新日期:2020-05-11
中文翻译:

au蛋白淀粉蛋白原纤维的摄取通过细胞病毒蛋白促进,并阻碍病毒在培养细胞中的繁殖。
关节病是普遍存在的致命性脑疾病,无法治愈。牛头颅病通过tau蛋白淀粉样蛋白的细胞间转移逐渐影响大脑,但其传播机制仍然未知。在这里,我们显示细胞病毒蛋白(PrP C)可能通过充当胞吞受体来促进培养细胞对tau聚集体的摄取。在小鼠神经母细胞瘤细胞中,下拉实验表明tau淀粉样蛋白与PrP C结合。用荧光标记的合成tau原纤维处理的野生型和PrP C敲除N2a细胞的共聚焦图像显示,在缺乏编码PrP C的基因的同基因细胞中,内化作用降低。用抗PrP C N近端表位的抗体预处理同一细胞会损害tau淀粉样蛋白的结合并降低其摄取。出乎意料的是,将长期感染ion病毒的细胞暴露于tau淀粉样蛋白会减少聚集的病毒蛋白的积累,这种作用在去除淀粉样蛋白后持续了超过72小时。这些结果表明这两种蛋白质之间存在双向相互作用:虽然PrP C介导tau纤维进入细胞,但PrP Sc的存在会大大减少其积累,这可能是由于pr病毒转化过程的损害。











































 京公网安备 11010802027423号
京公网安备 11010802027423号