当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The neuronal transcription factor Myt1L interacts via a conserved motif with the PAH1 domain of Sin3 to recruit the Sin3L/Rpd3L histone deacetylase complex
FEBS Letters ( IF 3.0 ) Pub Date : 2020-05-23 , DOI: 10.1002/1873-3468.13811 Ryan Dale Marcum 1 , Ishwar Radhakrishnan 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-05-23 , DOI: 10.1002/1873-3468.13811 Ryan Dale Marcum 1 , Ishwar Radhakrishnan 1
Affiliation
|
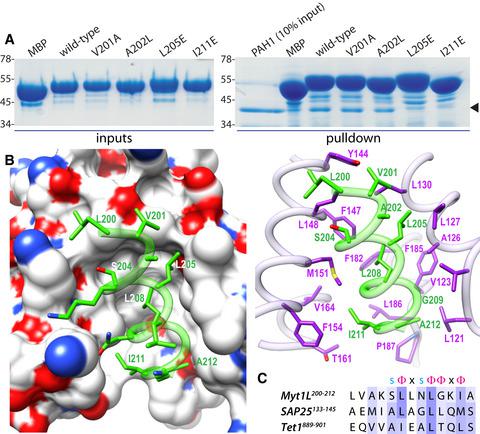
The Sin3L/Rpd3L histone deacetylase (HDAC) complex is one of six major HDAC complexes in the nucleus, and its recruitment by promoter‐bound transcription factors is an important step in many gene transcription regulatory pathways. Here, we investigate how the Myt1L zinc finger transcription factor, important for neuronal differentiation and the maintenance of neuronal identity, recruits this complex at the molecular level. We show that Myt1L, through a highly conserved segment shared with its paralogs, interacts directly and specifically with the Sin3 PAH1 domain, binding principally to the canonical hydrophobic cleft found in paired amphipathic helix domain (PAH) domains. Our findings are relevant not only for other members of the Myt family but also for enhancing our understanding of the rules of protein–protein interactions involving Sin3 PAH domains.
中文翻译:

神经元转录因子 Myt1L 通过一个保守基序与 Sin3 的 PAH1 结构域相互作用以招募 Sin3L/Rpd3L 组蛋白脱乙酰酶复合物
Sin3L/Rpd3L 组蛋白去乙酰化酶 (HDAC) 复合物是细胞核中六种主要的 HDAC 复合物之一,它通过启动子结合转录因子的募集是许多基因转录调控途径中的重要步骤。在这里,我们研究了 Myt1L 锌指转录因子(对神经元分化和维持神经元身份很重要)如何在分子水平上招募这种复合物。我们展示了 Myt1L,通过与其旁系同源物共享的高度保守的片段,直接和特异性地与 Sin3 PAH1 域相互作用,主要结合到成对的两亲螺旋域 (PAH) 域中发现的典型疏水裂缝。
更新日期:2020-05-23
中文翻译:

神经元转录因子 Myt1L 通过一个保守基序与 Sin3 的 PAH1 结构域相互作用以招募 Sin3L/Rpd3L 组蛋白脱乙酰酶复合物
Sin3L/Rpd3L 组蛋白去乙酰化酶 (HDAC) 复合物是细胞核中六种主要的 HDAC 复合物之一,它通过启动子结合转录因子的募集是许多基因转录调控途径中的重要步骤。在这里,我们研究了 Myt1L 锌指转录因子(对神经元分化和维持神经元身份很重要)如何在分子水平上招募这种复合物。我们展示了 Myt1L,通过与其旁系同源物共享的高度保守的片段,直接和特异性地与 Sin3 PAH1 域相互作用,主要结合到成对的两亲螺旋域 (PAH) 域中发现的典型疏水裂缝。











































 京公网安备 11010802027423号
京公网安备 11010802027423号