当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible Light-Catalyzed Benzylic C-H Bond Chlorination by a Combination of Organic Dye (Acr+-Mes) and N-Chlorosuccinimide.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-20 , DOI: 10.1021/acs.joc.0c01000 Ming Xiang 1, 2 , Chao Zhou 1, 2 , Xiu-Long Yang 1, 2 , Bin Chen 1, 2 , Chen-Ho Tung 1, 2 , Li-Zhu Wu 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-20 , DOI: 10.1021/acs.joc.0c01000 Ming Xiang 1, 2 , Chao Zhou 1, 2 , Xiu-Long Yang 1, 2 , Bin Chen 1, 2 , Chen-Ho Tung 1, 2 , Li-Zhu Wu 1, 2
Affiliation
|
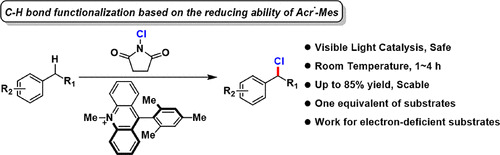
By combining “N-chlorosuccinimide (NCS)” as the safe chlorine source with “Acr+-Mes” as the photocatalyst, we successfully achieved benzylic C–H bond chlorination under visible light irradiation. Furthermore, benzylic chlorides could be converted to benzylic ethers smoothly in a one-pot manner by adding sodium methoxide. This mild and scalable chlorination method worked effectively for diverse toluene derivatives, especially for electron-deficient substrates. Careful mechanistic studies supported that NCS provided a hydrogen abstractor “N-centered succinimidyl radical,” which was responsible for the cleavage of the benzylic C–H bond, relying on the reducing ability of Acr•-Mes.
中文翻译:

通过有机染料(Acr + -Mes)和N-氯代琥珀酰亚胺的组合进行可见光催化的苯甲酰CH键氯化反应。
通过将作为安全氯源的“ N-氯琥珀酰亚胺(NCS)”与作为光催化剂的“ Acr + -Mes”相结合,我们在可见光照射下成功实现了苄基C–H键氯化。此外,通过加入甲醇钠可以一锅法将苄基氯平稳地转化为苄基醚。这种温和且可扩展的氯化方法可有效地用于多种甲苯衍生物,尤其是对于缺电子的底物。仔细的机理研究表明,NCS提供了一个氢抽象剂“ N-中心的琥珀酰亚胺基”,该基团依赖于Acr • -Mes的还原能力来裂解苄基的C–H键。
更新日期:2020-07-17
中文翻译:

通过有机染料(Acr + -Mes)和N-氯代琥珀酰亚胺的组合进行可见光催化的苯甲酰CH键氯化反应。
通过将作为安全氯源的“ N-氯琥珀酰亚胺(NCS)”与作为光催化剂的“ Acr + -Mes”相结合,我们在可见光照射下成功实现了苄基C–H键氯化。此外,通过加入甲醇钠可以一锅法将苄基氯平稳地转化为苄基醚。这种温和且可扩展的氯化方法可有效地用于多种甲苯衍生物,尤其是对于缺电子的底物。仔细的机理研究表明,NCS提供了一个氢抽象剂“ N-中心的琥珀酰亚胺基”,该基团依赖于Acr • -Mes的还原能力来裂解苄基的C–H键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号