当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantitative analysis of paracetamol, metacetamol, and orthocetamol in equine urine from racehorses in Japan using liquid chromatography-electrospray ionization-tandem mass spectrometry.
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1002/dta.2860 Hideaki Ishii 1, 2 , Taku Obara 2 , Kanichi Kusano 3 , Isao Kijima-Suda 1
Drug Testing and Analysis ( IF 2.9 ) Pub Date : 2020-06-24 , DOI: 10.1002/dta.2860 Hideaki Ishii 1, 2 , Taku Obara 2 , Kanichi Kusano 3 , Isao Kijima-Suda 1
Affiliation
|
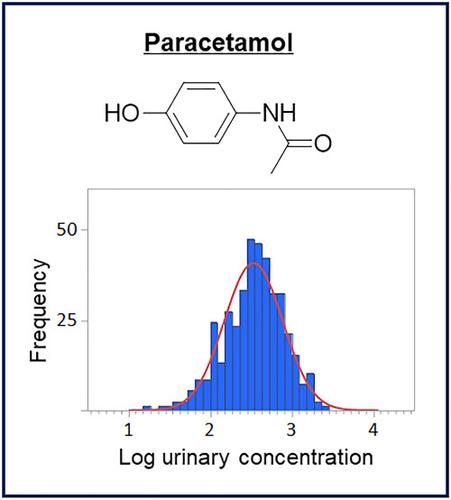
Paracetamol is commonly used as an over‐the‐counter analgesic and antipyretic medication for humans, but not sold as a legitimate therapeutic medication for horses in Japan. However, paracetamol is commonly found in horses together with its two isomers, metacetamol and orthocetamol. We previously reported that paracetamol and orthocetamol were both present in selected feed consumed by Japanese racehorses. For the purpose of the doping control of paracetamol in local Japanese horses, we proposed establishing residue limits (Japanese residue limits, JRLs) to minimize the risk of reporting paracetamol from environmental contributions and to differentiate its presence from active administration. Recently, we proposed a preliminary JRL for paracetamol in equine plasma based on a population study of more than 300 Japanese racehorses. In this paper, we will present our studies on the urinary concentrations of paracetamol, metacetamol, and orthocetamol in postrace samples collected from 403 Japanese racehorses over a 1 year period, detected using liquid chromatography–electrospray ionization–tandem mass spectrometry. Our results revealed that the hydrolyzed urinary concentrations of paracetamol, metacetamol, and orthocetamol were in the range 15.7–2,360 ng/mL (median 363 ng/mL), 8.07–382 ng/mL (84.5 ng/mL), and 919–74,418 ng/mL (13,475 ng/mL), respectively. Based on our statistical model, the preliminary JRL of hydrolyzed paracetamol in equine urine was determined to be 7,400 ng/mL, at a risk factor of 1 in 10,000. Further investigations will be required to demonstrate the applicability and validity of our preliminary plasma and urine JRLs to local Japanese racehorses.
中文翻译:

使用液相色谱-电喷雾电离串联质谱法定量分析日本赛马马尿中对乙酰氨基酚,扑热息痛和邻乙酰氨基酚的含量。
扑热息痛通常被用作人类的非处方镇痛和解热药,但在日本并未作为合法的马匹销售。然而,扑热息痛通常在马匹中与它的两种异构体,扑热息痛和邻苯二酚一起发现。我们以前曾报道过,日本赛马所食用的精选饲料中同时存在对乙酰氨基酚和邻苯二酚。为了控制对地马匹中扑热息痛的兴奋剂,我们建议建立残留限量(日本残留限量,JRL),以最大程度地减少对乙酰氨基酚从环境影响中报告的风险,并将其与主动给药区分开来。最近,我们根据对300多个日本赛马的种群研究,提出了马血浆中扑热息痛的初步JRL。在本文中,我们将介绍使用液相色谱-电喷雾电离-串联质谱法在1年内从403个日本赛马中收集的赛后样品中对乙酰氨基酚,扑热息痛和邻乙酚的尿液浓度的研究。我们的研究结果表明,对乙酰氨基酚,扑热息痛和邻苯二酚的水解尿液浓度范围为15.7–2,360 ng / mL(中位数363 ng / mL),8.07–382 ng / mL(84.5 ng / mL)和919–74,418 ng / mL(13,475 ng / mL)。根据我们的统计模型,马尿中水解对乙酰氨基酚的初步JRL被确定为7,400 ng / mL,风险系数为10,000。需要进行进一步的研究,以证明我们的初步血浆和尿液JRL在日本当地赛马中的适用性和有效性。
更新日期:2020-06-24
中文翻译:

使用液相色谱-电喷雾电离串联质谱法定量分析日本赛马马尿中对乙酰氨基酚,扑热息痛和邻乙酰氨基酚的含量。
扑热息痛通常被用作人类的非处方镇痛和解热药,但在日本并未作为合法的马匹销售。然而,扑热息痛通常在马匹中与它的两种异构体,扑热息痛和邻苯二酚一起发现。我们以前曾报道过,日本赛马所食用的精选饲料中同时存在对乙酰氨基酚和邻苯二酚。为了控制对地马匹中扑热息痛的兴奋剂,我们建议建立残留限量(日本残留限量,JRL),以最大程度地减少对乙酰氨基酚从环境影响中报告的风险,并将其与主动给药区分开来。最近,我们根据对300多个日本赛马的种群研究,提出了马血浆中扑热息痛的初步JRL。在本文中,我们将介绍使用液相色谱-电喷雾电离-串联质谱法在1年内从403个日本赛马中收集的赛后样品中对乙酰氨基酚,扑热息痛和邻乙酚的尿液浓度的研究。我们的研究结果表明,对乙酰氨基酚,扑热息痛和邻苯二酚的水解尿液浓度范围为15.7–2,360 ng / mL(中位数363 ng / mL),8.07–382 ng / mL(84.5 ng / mL)和919–74,418 ng / mL(13,475 ng / mL)。根据我们的统计模型,马尿中水解对乙酰氨基酚的初步JRL被确定为7,400 ng / mL,风险系数为10,000。需要进行进一步的研究,以证明我们的初步血浆和尿液JRL在日本当地赛马中的适用性和有效性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号