当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exo/endo stereocontrolled synthesis of spiroindoloindolizidines by using classical and microwave conditions via the 1,3‐dipolar cycloaddition reaction
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-20 , DOI: 10.1002/jhet.4005 Hamid Arvinnezhad 1 , Fatemeh Ghorbani 1 , Hormoz Khosravi 1 , Khosrow Jadidi 1 , Behrouz Notash 1 , Soheila Naderi 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-05-20 , DOI: 10.1002/jhet.4005 Hamid Arvinnezhad 1 , Fatemeh Ghorbani 1 , Hormoz Khosravi 1 , Khosrow Jadidi 1 , Behrouz Notash 1 , Soheila Naderi 1
Affiliation
|
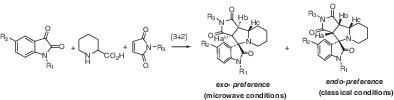
Using both classical reflux and microwave‐mediated conditions, a series of new spiroindoloindolizidines was synthesized by multicomponent 1,3‐dipolar cycloaddition of azomethine ylides in unprecedented exo /endo stereocontrolled. Both conditions easily afforded two identical and separable exo /endo diastereomeric ratios of cycloadducts. However, the ratio of two diastereomeric products obtained from conventional conditions was reversed in all examined cases when the reactions were explored under microwave‐mediated conditions. As expected, utilizing the microwave‐assisted conditions produced higher yields and reaction rates compared to classical conditions. The structure and exact stereochemistry of synthesized cycloadducts were determined by applying various 2D‐NMR spectroscopic techniques and single‐crystal X‐ray diffraction. Finally, the mechanism of the reaction has been briefly investigated by using density functional theory (DFT) calculations.
中文翻译:

通过经典条件和微波条件,通过1,3-偶极环加成反应,对exino / endo立体控制合成螺吲哚并吲哚并吡啶
在经典回流和微波介导条件下,通过空前的内/外立体控制,通过偶氮甲亚胺的多组分1,3-偶极环加成反应合成了一系列新的螺吲哚并吲哚并咪唑。两种情况都容易得到两个相同且可分离的exo / endo加合物的非对映体比率。但是,当在微波介导的条件下探索反应时,在所有检查的情况下,从常规条件获得的两种非对映异构体产物的比例都被反转。不出所料,与传统条件相比,利用微波辅助条件可产生更高的收率和反应速率。合成的环加合物的结构和确切的立体化学是通过应用各种2D-NMR光谱技术和单晶X射线衍射确定的。最后,通过使用密度泛函理论(DFT)计算简要地研究了反应机理。
更新日期:2020-05-20
中文翻译:

通过经典条件和微波条件,通过1,3-偶极环加成反应,对exino / endo立体控制合成螺吲哚并吲哚并吡啶
在经典回流和微波介导条件下,通过空前的内/外立体控制,通过偶氮甲亚胺的多组分1,3-偶极环加成反应合成了一系列新的螺吲哚并吲哚并咪唑。两种情况都容易得到两个相同且可分离的exo / endo加合物的非对映体比率。但是,当在微波介导的条件下探索反应时,在所有检查的情况下,从常规条件获得的两种非对映异构体产物的比例都被反转。不出所料,与传统条件相比,利用微波辅助条件可产生更高的收率和反应速率。合成的环加合物的结构和确切的立体化学是通过应用各种2D-NMR光谱技术和单晶X射线衍射确定的。最后,通过使用密度泛函理论(DFT)计算简要地研究了反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号