当前位置:
X-MOL 学术
›
Hum. Brain Mapp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deep learning-guided joint attenuation and scatter correction in multitracer neuroimaging studies.
Human Brain Mapping ( IF 4.8 ) Pub Date : 2020-05-21 , DOI: 10.1002/hbm.25039 Hossein Arabi 1 , Karin Bortolin 1 , Nathalie Ginovart 2, 3 , Valentina Garibotto 1, 4 , Habib Zaidi 1, 4, 5, 6
Human Brain Mapping ( IF 4.8 ) Pub Date : 2020-05-21 , DOI: 10.1002/hbm.25039 Hossein Arabi 1 , Karin Bortolin 1 , Nathalie Ginovart 2, 3 , Valentina Garibotto 1, 4 , Habib Zaidi 1, 4, 5, 6
Affiliation
|
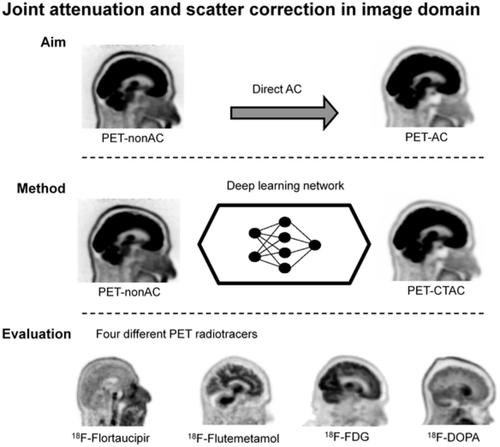
PET attenuation correction (AC) on systems lacking CT/transmission scanning, such as dedicated brain PET scanners and hybrid PET/MRI, is challenging. Direct AC in image‐space, wherein PET images corrected for attenuation and scatter are synthesized from nonattenuation corrected PET (PET‐nonAC) images in an end‐to‐end fashion using deep learning approaches (DLAC) is evaluated for various radiotracers used in molecular neuroimaging studies. One hundred eighty brain PET scans acquired using 18F‐FDG, 18F‐DOPA, 18F‐Flortaucipir (targeting tau pathology), and 18F‐Flutemetamol (targeting amyloid pathology) radiotracers (40 + 5, training/validation + external test, subjects for each radiotracer) were included. The PET data were reconstructed using CT‐based AC (CTAC) to generate reference PET‐CTAC and without AC to produce PET‐nonAC images. A deep convolutional neural network was trained to generate PET attenuation corrected images (PET‐DLAC) from PET‐nonAC. The quantitative accuracy of this approach was investigated separately for each radiotracer considering the values obtained from PET‐CTAC images as reference. A segmented AC map (PET‐SegAC) containing soft‐tissue and background air was also included in the evaluation. Quantitative analysis of PET images demonstrated superior performance of the DLAC approach compared to SegAC technique for all tracers. Despite the relatively low quantitative bias observed when using the DLAC approach, this approach appears vulnerable to outliers, resulting in noticeable local pseudo uptake and false cold regions. Direct AC in image‐space using deep learning demonstrated quantitatively acceptable performance with less than 9% absolute SUV bias for the four different investigated neuroimaging radiotracers. However, this approach is vulnerable to outliers which result in large local quantitative bias.
中文翻译:

多示踪剂神经影像研究中深度学习引导的联合衰减和散射校正。
在缺乏 CT/透射扫描的系统(例如专用脑部 PET 扫描仪和混合 PET/MRI)上进行 PET 衰减校正 (AC) 具有挑战性。图像空间中的直接 AC,其中使用深度学习方法 (DLAC) 以端到端方式从非衰减校正 PET (PET-nonAC) 图像合成经过衰减和散射校正的 PET 图像,对分子中使用的各种放射性示踪剂进行评估神经影像学研究。使用18 F-FDG、18 F-DOPA、18 F-Flortaucipir(针对 tau 病理学)和18 F-Frudemetamol(针对淀粉样蛋白病理学)放射性示踪剂(40 + 5,训练/验证 + 外部测试)获得 180 次脑部PET 扫描,每种放射性示踪剂的受试者)都包括在内。使用基于 CT 的 AC (CTAC) 重建 PET 数据以生成参考 PET-CTAC,而无需使用 AC 来生成 PET-nonAC 图像。训练深度卷积神经网络从 PET-nonAC 生成 PET 衰减校正图像 (PET-DLAC)。考虑从 PET-CTAC 图像获得的值作为参考,针对每种放射性示踪剂单独研究了该方法的定量准确性。评估中还包括包含软组织和背景空气的分段 AC 图(PET-SegAC)。PET 图像的定量分析表明,对于所有示踪剂,DLAC 方法均优于 SegAC 技术。尽管使用 DLAC 方法时观察到的定量偏差相对较低,但这种方法似乎容易受到异常值的影响,导致明显的局部伪吸收和假冷区域。使用深度学习在图像空间中进行直接 AC 展示了定量可接受的性能,对于四种不同的研究神经影像放射性示踪剂,绝对 SUV 偏差小于 9%。然而,这种方法很容易受到异常值的影响,从而导致较大的局部定量偏差。
更新日期:2020-05-21
中文翻译:

多示踪剂神经影像研究中深度学习引导的联合衰减和散射校正。
在缺乏 CT/透射扫描的系统(例如专用脑部 PET 扫描仪和混合 PET/MRI)上进行 PET 衰减校正 (AC) 具有挑战性。图像空间中的直接 AC,其中使用深度学习方法 (DLAC) 以端到端方式从非衰减校正 PET (PET-nonAC) 图像合成经过衰减和散射校正的 PET 图像,对分子中使用的各种放射性示踪剂进行评估神经影像学研究。使用18 F-FDG、18 F-DOPA、18 F-Flortaucipir(针对 tau 病理学)和18 F-Frudemetamol(针对淀粉样蛋白病理学)放射性示踪剂(40 + 5,训练/验证 + 外部测试)获得 180 次脑部PET 扫描,每种放射性示踪剂的受试者)都包括在内。使用基于 CT 的 AC (CTAC) 重建 PET 数据以生成参考 PET-CTAC,而无需使用 AC 来生成 PET-nonAC 图像。训练深度卷积神经网络从 PET-nonAC 生成 PET 衰减校正图像 (PET-DLAC)。考虑从 PET-CTAC 图像获得的值作为参考,针对每种放射性示踪剂单独研究了该方法的定量准确性。评估中还包括包含软组织和背景空气的分段 AC 图(PET-SegAC)。PET 图像的定量分析表明,对于所有示踪剂,DLAC 方法均优于 SegAC 技术。尽管使用 DLAC 方法时观察到的定量偏差相对较低,但这种方法似乎容易受到异常值的影响,导致明显的局部伪吸收和假冷区域。使用深度学习在图像空间中进行直接 AC 展示了定量可接受的性能,对于四种不同的研究神经影像放射性示踪剂,绝对 SUV 偏差小于 9%。然而,这种方法很容易受到异常值的影响,从而导致较大的局部定量偏差。



























 京公网安备 11010802027423号
京公网安备 11010802027423号