当前位置:
X-MOL 学术
›
Fungal Genet. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Localization of NPFxD motif-containing proteins in Aspergillus nidulans.
Fungal Genetics and Biology ( IF 2.4 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.fgb.2020.103412 Blake Commer 1 , Zachary Schultzhaus 1 , Brian D Shaw 1
Fungal Genetics and Biology ( IF 2.4 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.fgb.2020.103412 Blake Commer 1 , Zachary Schultzhaus 1 , Brian D Shaw 1
Affiliation
|
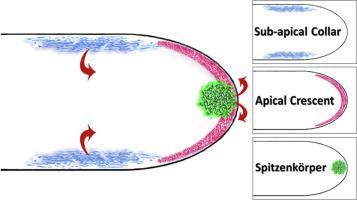
During growth, filamentous fungi produce polarized cells called hyphae. It is generally presumed that polarization of hyphae is dependent upon secretion through the Spitzenkörper, as well as a mechanism called apical recycling, which maintains a balance between the tightly coupled processes of endocytosis and exocytosis. Endocytosis predominates in an annular domain called the sub-apical endocytic collar, which is located in the region of plasma membrane 1-5 μm distal to the Spitzenkörper. It has previously been proposed that one function of the sub-apical endocytic collar is to maintain the apical localization of polarization proteins. These proteins mark areas of polarization at the apices of hyphae. However, as hyphae grow, these proteins are displaced along the membrane and some must then be removed at the sub-apical endocytic collar in order to maintain the hyphoid shape. While endocytosis is fairly well characterized in yeast, comparatively little is known about the process in filamentous fungi. Here, a bioinformatics approach was utilized to identify 39 Aspergillus nidulans proteins that are predicted to be cargo of endocytosis based on the presence of an NPFxD peptide motif. This motif is a necessary endocytic signal sequence first established in Saccharomyces cerevisiae, where it marks proteins for endocytosis through an interaction with the adapter protein Sla1p. It is hypothesized that some proteins that contain this NPFxD peptide sequence in A. nidulans will be potential targets for endocytosis, and therefore will localize either to the endocytic collar or to more proximal polarized regions of the cell, e.g. the apical dome or the Spitzenkörper. To test this, a subset of the motif-containing proteins in A. nidulans was tagged with GFP and the dynamic localization was evaluated. The documented localization patterns support the hypothesis that the motif marks proteins for localization to the polarized cell apex in growing hyphae.
中文翻译:

构巢曲霉中含有 NPFxD 基序的蛋白质的定位。
在生长过程中,丝状真菌产生称为菌丝的极化细胞。通常假设菌丝的极化取决于通过 Spitzenkörper 的分泌,以及一种称为顶端循环的机制,该机制在胞吞作用和胞吐作用的紧密耦合过程之间保持平衡。内吞作用主要存在于称为亚顶端内吞环的环形区域中,该区域位于 Spitzenkörper 远端 1-5 μm 的质膜区域。先前已经提出,亚顶端内吞颈圈的一项功能是维持极化蛋白的顶端定位。这些蛋白质标记了菌丝顶端的极化区域。然而,随着菌丝的生长,这些蛋白质沿细胞膜移动,然后必须在根尖下的内吞环处去除一些蛋白质,以保持菌丝状。虽然内吞作用在酵母中得到了很好的表征,但对丝状真菌中的过程知之甚少。在这里,利用生物信息学方法来识别 39 种构巢曲霉蛋白,根据 NPFxD 肽基序的存在,这些蛋白被预测为内吞作用。该基序是首先在酿酒酵母中建立的必要内吞信号序列,它通过与衔接蛋白 Sla1p 的相互作用标记用于内吞作用的蛋白质。假设在构巢曲霉中包含此 NPFxD 肽序列的一些蛋白质将成为内吞作用的潜在目标,因此将定位到细胞内吞环或更近端的极化区域,例如顶端圆顶或 Spitzenkörper。为了测试这一点,构巢曲霉中含有基序的蛋白质的一个子集用 GFP 标记,并评估了动态定位。记录的定位模式支持这样的假设,即该基序标记蛋白质以定位到生长的菌丝中的极化细胞顶点。
更新日期:2020-05-21
中文翻译:

构巢曲霉中含有 NPFxD 基序的蛋白质的定位。
在生长过程中,丝状真菌产生称为菌丝的极化细胞。通常假设菌丝的极化取决于通过 Spitzenkörper 的分泌,以及一种称为顶端循环的机制,该机制在胞吞作用和胞吐作用的紧密耦合过程之间保持平衡。内吞作用主要存在于称为亚顶端内吞环的环形区域中,该区域位于 Spitzenkörper 远端 1-5 μm 的质膜区域。先前已经提出,亚顶端内吞颈圈的一项功能是维持极化蛋白的顶端定位。这些蛋白质标记了菌丝顶端的极化区域。然而,随着菌丝的生长,这些蛋白质沿细胞膜移动,然后必须在根尖下的内吞环处去除一些蛋白质,以保持菌丝状。虽然内吞作用在酵母中得到了很好的表征,但对丝状真菌中的过程知之甚少。在这里,利用生物信息学方法来识别 39 种构巢曲霉蛋白,根据 NPFxD 肽基序的存在,这些蛋白被预测为内吞作用。该基序是首先在酿酒酵母中建立的必要内吞信号序列,它通过与衔接蛋白 Sla1p 的相互作用标记用于内吞作用的蛋白质。假设在构巢曲霉中包含此 NPFxD 肽序列的一些蛋白质将成为内吞作用的潜在目标,因此将定位到细胞内吞环或更近端的极化区域,例如顶端圆顶或 Spitzenkörper。为了测试这一点,构巢曲霉中含有基序的蛋白质的一个子集用 GFP 标记,并评估了动态定位。记录的定位模式支持这样的假设,即该基序标记蛋白质以定位到生长的菌丝中的极化细胞顶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号