Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.actbio.2020.05.016 I Geremia 1 , D Pavlenko 1 , K Maksymow 2 , M Rüth 2 , H D Lemke 2 , D Stamatialis 1
|
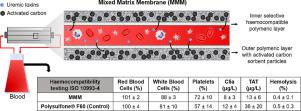
The patients with end stage kidney disease need haemodialysis therapies, using an artificial kidney. Nevertheless, the current therapies cannot remove a broad range of uremic toxins compared to the natural kidney. Adsorption therapies, using sorbent-based columns, can improve the clearance of uremic toxins, but the sorbent particles often require polymeric coatings to improve their haemocompatibility leading to mass transfer limitations and to lowering of their performance.
Earlier, we have developed a dual layer Mixed Matrix fiber Membrane (MMM) based on polyethersulfone/polyvinylpyrrolidone (PES/PVP) polymer blends. There, the sorbent activated carbon particles are embedded in the outer membrane layer for achieving higher removal whereas the inner blood contacting selective membrane layer should achieve optimal blood compatibility. In this work, we evaluate in detail the haemocompatibility of the MMM following the norm ISO 10993–4. We study two generations of MMM having different dimensions and transport characteristics; one with low flux and no albumin leakage and another with high flux but some albumin leakage. The results are compared to those of home-made PES/PVP single layer hollow fiber and to various control fibers already applied in the clinic. Our results show that the low flux MMM successfully avoids contact of blood with the activated carbon and has good haemocompatibility, comparable to membranes currently used in the clinic.
Statement of Significance
Haemodialysis is a life-sustaining extracorporeal treatment for renal disease, however a broad range of uremic toxins cannot still be removed. In our previous works we showed that a double layer Mixed Matrix Membrane (MMM) composed of polyethersulfone/polyvinylpyrrolidone and activated carbon can achieve higher removal of uremic toxics compared to commercial haemodialysers. In this work we evaluate the haemocompatibility profile of the MMM in order to facilitate its clinical implementation. The lumen particle-free layer of the MMM successfully avoids the contact of blood with the poorly blood-compatible activated carbon. Moreover, thanks to the high amount of polyvinylpyrrolidone and to the smoothness of the lumen layer, the MMM has very good haemocompatibility, comparable to membranes currently used in the clinic.
中文翻译:

混合基质血液透析膜血液相容性的离体评估。
患有晚期肾脏疾病的患者需要使用人造肾脏进行血液透析疗法。然而,与天然肾脏相比,目前的疗法无法去除广泛的尿毒症毒素。使用基于吸附剂的色谱柱的吸附疗法可以提高尿毒症毒素的清除率,但是吸附剂颗粒通常需要聚合物涂层才能改善其血液相容性,从而导致传质受限并降低其性能。
之前,我们已经开发了基于聚醚砜/聚乙烯吡咯烷酮(PES / PVP)聚合物共混物的双层混合基质纤维膜(MMM)。在那里,吸附剂活性炭颗粒嵌入外膜层中,以实现更高的去除率,而与内血接触的选择性膜层应达到最佳的血液相容性。在这项工作中,我们将按照ISO 10993-4规范详细评估MMM的血液相容性。我们研究了具有不同尺寸和运输特性的两代MMM。一种是低通量,无白蛋白泄漏,另一种是高通量,但有白蛋白泄漏。将结果与自制的PES / PVP单层中空纤维的结果以及已经在临床中应用的各种对照纤维的结果进行比较。
重要声明
血液透析是肾脏疾病的一种维持生命的体外治疗,但是仍然不能清除广泛的尿毒症毒素。在我们以前的工作中,我们表明与商业血液透析仪相比,由聚醚砜/聚乙烯吡咯烷酮和活性炭组成的双层混合基质膜(MMM)可以实现更高的尿毒症毒性去除。在这项工作中,我们评估MMM的血液相容性,以促进其临床应用。MMM的无内腔颗粒层可成功避免血液与血液相容性差的活性炭接触。而且,由于大量的聚乙烯吡咯烷酮和管腔层的光滑性,MMM具有非常好的血液相容性,与目前临床上使用的膜相当。



























 京公网安备 11010802027423号
京公网安备 11010802027423号