Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.actbio.2020.05.022 Nazia Mehrban 1 , Catalina Pineda Molina 2 , Lina M Quijano 2 , James Bowen 3 , Scott A Johnson 2 , Joseph Bartolacci 4 , Jordan T Chang 5 , David A Scott 6 , Derek N Woolfson 7 , Martin A Birchall 8 , Stephen F Badylak 9
|
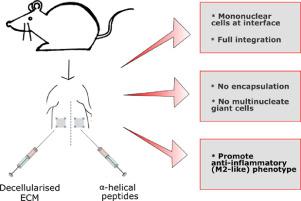
Tissue engineering materials play a key role in how closely the complex architectural and functional characteristics of native healthy tissue can be replicated. Traditional natural and synthetic materials are superseded by bespoke materials that cross the boundary between these two categories. Here we present hydrogels that are derived from decellularised extracellular matrix and those that are synthesised from de novo α-helical peptides. We assess in vitro activation of murine macrophages to our hydrogels and whether these gels induce an M1-like or M2-like phenotype. This was followed by the in vivo immune macrophage response to hydrogels injected into rat partial-thickness abdominal wall defects. Over 28 days we observe an increase in mononuclear cell infiltration at the hydrogel-tissue interface without promoting a foreign body reaction and see no evidence of hydrogel encapsulation or formation of multinucleate giant cells. We also note an upregulation of myogenic differentiation markers and the expression of anti-inflammatory markers Arginase1, IL-10, and CD206, indicating pro-remodelling for all injected hydrogels. Furthermore, all hydrogels promote an anti-inflammatory environment after an initial spike in the pro-inflammatory phenotype. No difference between the injected site and the healthy tissue is observed after 28 days, indicating full integration. These materials offer great potential for future applications in regenerative medicine and towards unmet clinical needs.
Statement of Significance
Materials play a key role in how closely the complex architectural and functional characteristics of native healthy tissue can be replicated in tissue engineering. Here we present injectable hydrogels derived from decellularised extracellular matrix and de novo designed α-helical peptides. Over 28 days in the rat abdominal wall we observe an increase in mononuclear cell infiltration at the hydrogel-tissue interface with no foreign body reaction, no evidence of hydrogel encapsulation and no multinucleate giant cells. Our data indicate pro-remodelling and the promotion of an anti-inflammatory environment for all injected hydrogels with evidence of full integration with healthy tissue after 28 days. These unique materials offer great potential for future applications in regenerative medicine and towards designing materials for unmet clinical needs.
中文翻译:

宿主巨噬细胞对源自ECM和α-螺旋肽的可注射水凝胶的反应。
组织工程材料在可复制天然健康组织的复杂结构和功能特性方面起着关键作用。跨越这两个类别边界的定制材料将取代传统的天然和合成材料。在这里,我们介绍了衍生自脱细胞的细胞外基质的水凝胶和从头α-螺旋肽合成的水凝胶。我们评估了小鼠巨噬细胞对我们的水凝胶的体外活化,以及这些凝胶是否诱导了M1或M2样表型。其次是体内免疫巨噬细胞对注射到大鼠部分厚度腹壁缺损的水凝胶的反应。在超过28天的时间内,我们观察到在水凝胶-组织界面处单核细胞浸润的增加,而没有促进异物反应,并且没有发现水凝胶包封或形成多核巨细胞的证据。我们还注意到成肌分化标志物和抗炎标志物Arginase1,IL-10和CD206的表达上调,表明所有注射的水凝胶都进行了重塑。此外,所有的水凝胶在促炎表型开始达到峰值后都会促进抗炎环境。28天后未观察到注射部位与健康组织之间的差异,表明完全整合。
重要声明
材料在组织工程中可以如何复制天然健康组织的复杂结构和功能特性方面起着关键作用。在这里,我们介绍从脱细胞的细胞外基质和从头衍生的可注射水凝胶设计的α-螺旋肽。在大鼠腹壁超过28天,我们观察到水凝胶-组织界面处单核细胞浸润的增加,没有异物反应,没有水凝胶包封的证据,也没有多核巨细胞。我们的数据表明所有注射的水凝胶均具有促重塑作用,并促进了抗炎环境的发展,并在28天后与健康组织完全融合。这些独特的材料为再生医学的未来应用和设计满足未满足临床需求的材料提供了巨大的潜力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号