当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Menthol-Based Deep Eutectic Solvent Dispersive Liquid–Liquid Microextraction: A Simple and Quick Approach for the Analysis of Phthalic Acid Esters from Water and Beverage Samples
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-05-19 , DOI: 10.1021/acssuschemeng.0c02603 Cecilia Ortega-Zamora 1 , Javier González-Sálamo 1, 2 , Cintia Hernández-Sánchez 2, 3 , Javier Hernández-Borges 1, 2
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-05-19 , DOI: 10.1021/acssuschemeng.0c02603 Cecilia Ortega-Zamora 1 , Javier González-Sálamo 1, 2 , Cintia Hernández-Sánchez 2, 3 , Javier Hernández-Borges 1, 2
Affiliation
|
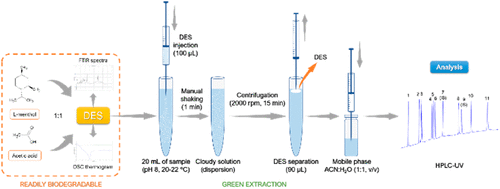
In this work, a natural deep eutectic solvent (NADES) composed of l-menthol:acetic acid 1:1 (molar ratio) was applied for the first time as the extraction solvent for the dispersive liquid–liquid microextraction (DLLME) of a group of 9 phthalic acid esters (i.e., dipropyl phthalate, DPP; butyl benzyl phthalate, BBP; dibutyl phthalate, DBP; diisopentyl phthalate, DIPP; di-n-pentyl phthalate, DNPP; dicyclohexyl phthalate, DCHP; di(2-ethylhexyl) phthalate, DEHP; diisononyl phthalate, DINP; and diisodecyl phthalate, DIDP) from different water samples (i.e., tap and mineral water) and an apple juice beverage. Dihexyl phthalate (DHP) and di-n-octyl phthalate (DNOP) were used as internal standards. After addition of the DES, agitation, and centrifugation, the NADES droplet was collected, dissolved in the mobile phase, and injected in a high-performance liquid chromatograph coupled to a UV detector. Relative recovery percentages were in the range 71–120% with relative standard deviation values ≤20%, while the limits of quantification of the method were in the range 3.6–22 μg/L for tap water, 3.8–20 μg/L for mineral water, and 4.0–23 μg/L for an apple juice drink. Infrared spectroscopy and differential scanning calorimetry analysis of the DES drop after the DLLME procedure revealed the absence of the DES after the extraction and the existence of an l-menthol-rich phase as a result of an important transfer of acetic acid to the aqueous phase. However, the use of l-menthol alone, which required the previous thermal solubilization of l-menthol, was not able to quantitatively extract some of the target analytes. The extraction carried out after the thermal solubilization of l-menthol and the addition of an acid like HCl suggested that the extraction capacity of the DES is caused by a “transient network” originated by the acidity of the medium, independently of the nature of the acid. The optimized NADES-DLLME procedure was extremely simple, quick, and also environmentally friendly, as a result of the biodegradability and relatively low toxicity of both components of the DES.
中文翻译:

基于薄荷醇的深共熔溶剂分散液-液微萃取:一种简单快速的方法,用于分析水和饮料样品中的邻苯二甲酸酯
在这项工作中,首次将由l-薄荷醇:乙酸1:1(摩尔比)组成的天然深共熔溶剂(NADES)用作一组分散液-液微萃取(DLLME)的萃取溶剂。 9种邻苯二甲酸酯(即邻苯二甲酸二丙酯DPP;邻苯二甲酸丁苄酯BBP;邻苯二甲酸二丁酯DBP;邻苯二甲酸二异戊酯DIPP;邻苯二甲酸二正戊酯DNPP;邻苯二甲酸二环己基酯DCHP;邻苯二甲酸二(2-乙基己基)酯,DEHP,邻苯二甲酸二异壬酯,DINP和邻苯二甲酸二异癸酯(DIDP),分别来自不同的水样品(即自来水和矿泉水)和苹果汁饮料。二己酯(DHP)和二- Ñ邻苯二甲酸正辛酯(DNOP)用作内标。加入DES,搅拌和离心后,收集NADES液滴,将其溶解在流动相中,然后注入连接有UV检测器的高效液相色谱仪中。相对回收率在71–120%的范围内,相对标准偏差值≤20%,而该方法的定量限在自来水的范围为3.6–22μg/ L,在矿物质中的范围为3.8–20μg/ L水和4.0–23μg/ L的苹果汁饮料。DLLME程序后对DES滴的红外光谱和差示扫描量热分析表明,提取后不存在DES,并且存在l乙酸大量转移到水相中的结果是富含薄荷醇的相。但是,仅使用1-薄荷醇需要先前的1-薄荷醇的热增溶,因此不能定量地提取一些目标分析物。在对1-薄荷醇进行热增溶并添加酸(如HCl)后进行的萃取表明,DES的萃取能力是由“瞬态网络”引起的,该“瞬态网络”是由介质的酸度引起的,而与介质的性质无关。酸。由于DES两种成分的生物降解性和相对较低的毒性,因此优化的NADES-DLLME程序极其简单,快速且对环境友好。
更新日期:2020-05-19
中文翻译:

基于薄荷醇的深共熔溶剂分散液-液微萃取:一种简单快速的方法,用于分析水和饮料样品中的邻苯二甲酸酯
在这项工作中,首次将由l-薄荷醇:乙酸1:1(摩尔比)组成的天然深共熔溶剂(NADES)用作一组分散液-液微萃取(DLLME)的萃取溶剂。 9种邻苯二甲酸酯(即邻苯二甲酸二丙酯DPP;邻苯二甲酸丁苄酯BBP;邻苯二甲酸二丁酯DBP;邻苯二甲酸二异戊酯DIPP;邻苯二甲酸二正戊酯DNPP;邻苯二甲酸二环己基酯DCHP;邻苯二甲酸二(2-乙基己基)酯,DEHP,邻苯二甲酸二异壬酯,DINP和邻苯二甲酸二异癸酯(DIDP),分别来自不同的水样品(即自来水和矿泉水)和苹果汁饮料。二己酯(DHP)和二- Ñ邻苯二甲酸正辛酯(DNOP)用作内标。加入DES,搅拌和离心后,收集NADES液滴,将其溶解在流动相中,然后注入连接有UV检测器的高效液相色谱仪中。相对回收率在71–120%的范围内,相对标准偏差值≤20%,而该方法的定量限在自来水的范围为3.6–22μg/ L,在矿物质中的范围为3.8–20μg/ L水和4.0–23μg/ L的苹果汁饮料。DLLME程序后对DES滴的红外光谱和差示扫描量热分析表明,提取后不存在DES,并且存在l乙酸大量转移到水相中的结果是富含薄荷醇的相。但是,仅使用1-薄荷醇需要先前的1-薄荷醇的热增溶,因此不能定量地提取一些目标分析物。在对1-薄荷醇进行热增溶并添加酸(如HCl)后进行的萃取表明,DES的萃取能力是由“瞬态网络”引起的,该“瞬态网络”是由介质的酸度引起的,而与介质的性质无关。酸。由于DES两种成分的生物降解性和相对较低的毒性,因此优化的NADES-DLLME程序极其简单,快速且对环境友好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号