当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of Enantioenriched β-Amino Secondary Amides via an Enantioselective [4+2] Cycloaddition Reaction of Vinyl Azides with N-Acyl Imines Catalyzed by a Chiral Brønsted Acid.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-05-19 , DOI: 10.1002/chem.202002049 Taishi Nakanishi 1 , Jun Kikuchi 1 , Atsushi Kaga 2 , Shunsuke Chiba 2 , Masahiro Terada 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-05-19 , DOI: 10.1002/chem.202002049 Taishi Nakanishi 1 , Jun Kikuchi 1 , Atsushi Kaga 2 , Shunsuke Chiba 2 , Masahiro Terada 1
Affiliation
|
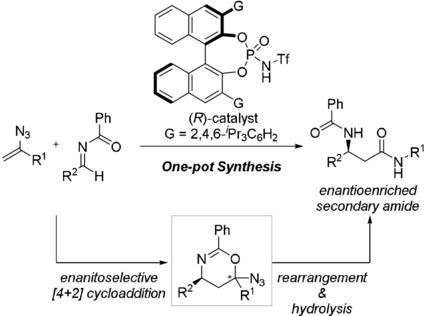
A catalytic enantioselective synthesis of β‐amino secondary amides was achieved using vinyl azides as the enamine‐type nucleophile and chiral N‐Tf phosphoramide as the chiral Brønsted acid catalyst through a five‐step sequential transformation in one pot. The established sequential transformation involves an enantioselective [4+2] cycloaddition reaction of vinyl azides with N‐acyl imines as the key stereo‐determining step that is efficiently accelerated by a chiral N‐Tf phosphoramide catalyst in a highly enantioselective manner in most cases. Further generation of the iminodiazonium ion intermediate through ring opening of the cycloaddition product and subsequent skeletal rearrangement involving Schmidt‐type 1,2‐aryl group migration followed by recyclization of the resulting nitrilium ion were also initiated by the same acid catalyst. Final acid hydrolysis of the recyclized products in the same pot gave rise to enantioenriched β‐amino amides through C−C bond formation at the α‐position of the secondary amides.
中文翻译:

通过手性布朗斯台德酸催化的乙烯基叠氮化物与N-酰基亚胺的对映选择性[4 + 2]环加成反应,一锅合成富对映体的β-氨基仲酰胺。
通过在一个罐中进行五步顺序转化,将乙烯基叠氮化物用作烯胺型亲核试剂,将手性N- Tf磷酰胺用作手性布朗斯台德酸催化剂,实现了β-氨基仲酰胺的催化对映选择性合成。建立的顺序转化涉及叠氮化物与N-酰基亚胺的对映选择性[4 + 2]环加成反应,这是关键的立体确定步骤,可通过手性N来有效地加速‐Tf磷酰胺催化剂在大多数情况下都具有高度对映选择性。通过环加成产物的开环和随后涉及施密特型1,2-芳基迁移的骨架重排以及随后的腈离子的再环化,也进一步引发了亚氨基重氮离子中间体的生成。在同一罐中对循环产物进行最终的酸水解,通过在仲酰胺的α位形成C-C键,产生了对映体富集的β-氨基酰胺。
更新日期:2020-07-02
中文翻译:

通过手性布朗斯台德酸催化的乙烯基叠氮化物与N-酰基亚胺的对映选择性[4 + 2]环加成反应,一锅合成富对映体的β-氨基仲酰胺。
通过在一个罐中进行五步顺序转化,将乙烯基叠氮化物用作烯胺型亲核试剂,将手性N- Tf磷酰胺用作手性布朗斯台德酸催化剂,实现了β-氨基仲酰胺的催化对映选择性合成。建立的顺序转化涉及叠氮化物与N-酰基亚胺的对映选择性[4 + 2]环加成反应,这是关键的立体确定步骤,可通过手性N来有效地加速‐Tf磷酰胺催化剂在大多数情况下都具有高度对映选择性。通过环加成产物的开环和随后涉及施密特型1,2-芳基迁移的骨架重排以及随后的腈离子的再环化,也进一步引发了亚氨基重氮离子中间体的生成。在同一罐中对循环产物进行最终的酸水解,通过在仲酰胺的α位形成C-C键,产生了对映体富集的β-氨基酰胺。









































 京公网安备 11010802027423号
京公网安备 11010802027423号