当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleophilic RhI Catalyzed Selective Isomerization of Terminal Aziridines to Enamides
ChemCatChem ( IF 3.8 ) Pub Date : 2020-05-20 , DOI: 10.1002/cctc.202000597 Yingying Tian 1 , Doris Kunz 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-05-20 , DOI: 10.1002/cctc.202000597 Yingying Tian 1 , Doris Kunz 1
Affiliation
|
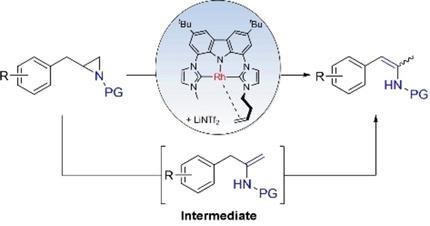
The selective isomerization of various terminal N‐Boc protected aziridines to enamides was realized using the highly reactive nucleophilic rhodium catalyst C with the Lewis acid LiNTf2 as co‐catalyst under moderate conditions. The reaction proceeds smoothly with only 1 mol% catalyst loading and excellent yields were achieved. An intermediate containing an enamide with a non‐conjugated terminal C=C double bond was detected during the course of the reaction, which isomerizes to form the thermodynamically favored 2‐amido styrene. Mechanistic insight is gained based on these observations.
中文翻译:

亲核RhI催化末端氮丙啶选择性异构化成酰胺。
使用高反应性亲核铑催化剂C和路易斯酸LiNTf 2作为助催化剂,在中等条件下实现了各种N - Boc末端保护的氮丙啶选择性异构化为酰胺。反应仅以1mol%的催化剂负载而顺利进行,并且获得了优异的产率。在反应过程中检测到一种中间体,该中间体含有一个非共轭末端C = C双键的酰胺,该中间体异构化形成热力学上较受欢迎的2-氨基苯乙烯。基于这些观察结果获得了机械的见解。
更新日期:2020-05-20
中文翻译:

亲核RhI催化末端氮丙啶选择性异构化成酰胺。
使用高反应性亲核铑催化剂C和路易斯酸LiNTf 2作为助催化剂,在中等条件下实现了各种N - Boc末端保护的氮丙啶选择性异构化为酰胺。反应仅以1mol%的催化剂负载而顺利进行,并且获得了优异的产率。在反应过程中检测到一种中间体,该中间体含有一个非共轭末端C = C双键的酰胺,该中间体异构化形成热力学上较受欢迎的2-氨基苯乙烯。基于这些观察结果获得了机械的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号