当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of C1−C9 and C10−C21 Fragments of (−)‐Dolabriferol
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-05-20 , DOI: 10.1002/ajoc.202000255 Anandaraju Bandaru 1 , Debjani Si 1 , Krishna P. Kaliappan 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-05-20 , DOI: 10.1002/ajoc.202000255 Anandaraju Bandaru 1 , Debjani Si 1 , Krishna P. Kaliappan 1
Affiliation
|
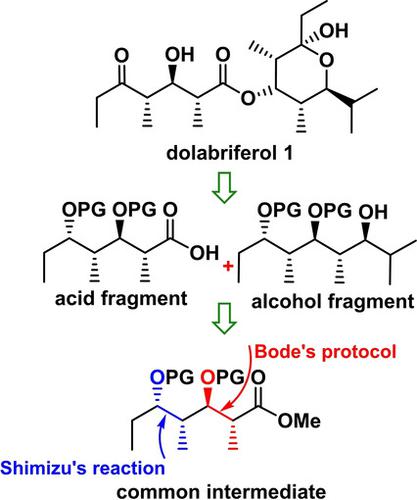
Our efforts on the synthesis of C1−C9 and C10−C21 subunits of dolabriferol, a non‐contiguous polypropionate marine natural product, from a common intermediate using two non‐aldol variants is reported. i) Shimizu reaction, a Pd(0) mediated stereoselective epoxy‐ring opening of alkenyl oxiranes, has been employed for the stereoselective installation of methyl groups at C4 and C13 and ii) Bode's protocol, a NHC‐mediated reaction on β‐epoxy aldehydes, has been utilized for stereoselective construction of methyl and hydroxyl groups in anti‐fashion at C6−C7 and C15−C16 in the natural product.
中文翻译:

(-)-二十碳四烯醇的C1-C9和C10-C21片段的合成
据报道,我们使用两种非醛醇变体从共同的中间体合成多布拉夫罗酚的C 1 -C 9和C 10 -C 21亚基,这是一种不连续的聚丙烯酸酯海洋天然产物。i)清水反应(Pd(0)介导的烯基环氧乙烷的立体选择性环氧环开环)已用于在C 4和C 13处进行甲基的立体选择性安装,ii)Bode方案,NHC介导的β-上的反应环氧醛已被用于在C 6 -C 7和C 15 -C 16的反时尚立体选择性地构建甲基和羟基 在天然产物中。
更新日期:2020-07-08
中文翻译:

(-)-二十碳四烯醇的C1-C9和C10-C21片段的合成
据报道,我们使用两种非醛醇变体从共同的中间体合成多布拉夫罗酚的C 1 -C 9和C 10 -C 21亚基,这是一种不连续的聚丙烯酸酯海洋天然产物。i)清水反应(Pd(0)介导的烯基环氧乙烷的立体选择性环氧环开环)已用于在C 4和C 13处进行甲基的立体选择性安装,ii)Bode方案,NHC介导的β-上的反应环氧醛已被用于在C 6 -C 7和C 15 -C 16的反时尚立体选择性地构建甲基和羟基 在天然产物中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号