Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-05-20 , DOI: 10.1016/j.jmb.2020.05.009 Shuang Li 1 , Shixuan Liu 1 , Yihu Yang 1 , Weikai Li 1
|
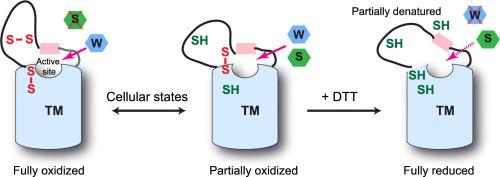
Intramembrane enzymes are often difficult for biochemical characterization. Human vitamin K epoxide reductase (VKOR) is the target of warfarin. However, this intramembrane enzyme becomes insensitive to warfarin inhibition in vitro, preventing the characterization of inhibition kinetics for decades. Here we employ structural biology methods to identify stable VKOR and VKOR-like proteins and purify them to near homogeneity. We find that the key to maintain their warfarin sensitivity is to stabilize their native protein conformation in vitro. Reduced glutathione drastically increases the warfarin sensitivity of a VKOR-like protein from Takifugu rubripes, presumably through maintaining a disulfide-bonded conformation. Effective inhibition of human VKOR-like requires also the use of LMNG, a mild detergent developed for crystallography to increase membrane protein stability. Human VKOR needs to be preserved in ER-enriched microsomes to exhibit warfarin sensitivity, whereas human VKOR purified in LMNG is stable only with pre-bound warfarin. Under these optimal conditions, warfarin inhibits with tight-binding kinetics. Overall, our studies show that structural biology methods are ideal for stabilizing intramembrane enzymes. Optimizing toward their inhibitor-binding conformation enables the characterization of enzyme kinetics in difficult cases.
中文翻译:

华法林抑制动力学的表征需要稳定膜内维生素 K 环氧化物还原酶。
膜内酶通常难以进行生化表征。人维生素 K 环氧化物还原酶 (VKOR) 是华法林的靶点。然而,这种膜内酶在体外对华法林抑制变得不敏感,阻止了抑制动力学的表征数十年。在这里,我们采用结构生物学方法来鉴定稳定的 VKOR 和 VKOR 样蛋白,并将它们纯化至接近同质。我们发现维持其华法林敏感性的关键是在体外稳定其天然蛋白质构象。减少的谷胱甘肽显着增加了来自Takifugu rubripes的 VKOR 样蛋白的华法林敏感性,大概是通过保持二硫键的构象。对人类 VKOR 样的有效抑制还需要使用 LMNG,这是一种为晶体学开发的温和洗涤剂,可增加膜蛋白的稳定性。人类 VKOR 需要保存在富含 ER 的微粒体中才能表现出对华法林的敏感性,而在 LMNG 中纯化的人类 VKOR 仅与预先结合的华法林一起稳定。在这些最佳条件下,华法林以紧密结合的动力学进行抑制。总的来说,我们的研究表明结构生物学方法是稳定膜内酶的理想选择。优化它们的抑制剂结合构象可以在困难的情况下表征酶动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号