International Journal of Greenhouse Gas Control ( IF 4.6 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.ijggc.2020.103061 Mayuri Gupta , Hallvard F. Svendsen
|
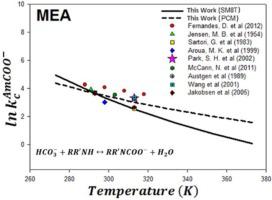
Thermodynamic properties and carbamate stability constants (Kc) for a dataset of 25 amines and alkanolamines, with desirable post combustion CO2 capture (PCC) solvent properties, have been studied extensively employing various gaseous phase calculations and solvation models. A comprehensive study of gaseous phase free energy and enthalpy is carried out using density functional methods [B3LYP/6-311++G(d,p)], and composite methods (G3MP2B3, G3MP2, G4MP2 and CBS-QB3). Implicit solvation models (PCM, SM8T and DivCon) and the Explicit Solvation Shell Model (ESS) were used to study solvation free energy of various neutral and ionic species present in the amine-carbamate formation reaction. Temperature dependent carbamate stability constants are calculated for the carbamate formation reaction of amines and alkanolamines to better understand the effect of temperature on temperature swing absorption-desorption PCC processes. The temperature dependency of Kc was compared against available experimental data.
中文翻译:

建模与温度相关的胺的绝对氨基甲酸酯稳定性常数和温度相关性,用于CO 2捕集
25种胺和链烷醇胺的数据集的热力学性质和氨基甲酸酯稳定常数(Kc),并具有理想的燃烧后CO 2捕集(PCC)溶剂的性质,已广泛使用各种气相计算和溶剂化模型进行了研究。使用密度泛函方法[B3LYP / 6-311 ++ G(d,p)]和复合方法(G3MP2B3,G3MP2,G4MP2和CBS-QB3)对气相自由能和焓进行了全面研究。隐式溶剂化模型(PCM,SM8T和DivCon)和显式溶剂化壳模型(ESS)用于研究胺-氨基甲酸酯形成反应中存在的各种中性和离子性物质的溶剂化自由能。计算胺和链烷醇胺的氨基甲酸酯形成反应的温度依赖性氨基甲酸酯稳定性常数,以更好地理解温度对变温吸收-解吸PCC工艺的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号