Chemical Engineering Research and Design ( IF 3.7 ) Pub Date : 2020-05-20 , DOI: 10.1016/j.cherd.2020.04.026 Nedasadat Saadati Ardestani , Mitra Amani , Leila Moharrery
|
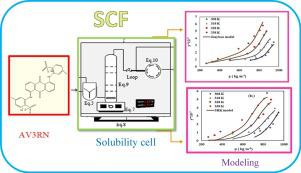
In this study, the solubility of Anthraquinone Violet 3RN (AV3RN) was measured in supercritical CO2 with/without methanol as co-solvent at different temperature (308, 318, 328 and 338) K and pressures up to 34.0 MPa. The solubility of AV3RN varied in the range of 0.047 × 10−5 to 0.546 × 10−5 mole fractions while its solubility by co-solvent ranged from 0.44 × 10−5 to 5.77 × 10−5 mole fractions. Calculated enhanced solubility factors are in the range of 7.7–15.9 by addition of 6% mole methanol as co-solvent. For both studied systems, AV3RN solubility was correlated with several density-based models and equations of state (EoSs) including Peng–Robinson (PR) and Soave–Redlich–Kwong (SRK). Empirical models developed by Khansary et al. along with Garlapati–Madras models in binary system, and the Jouyban et al. model in ternary system exhibited the highest accordance with experimental data. Also it was shown that, the PR model performed better in predicting the solubility of AV3RN when compared with SRK.
中文翻译:

在有/无助溶剂下测定蒽醌紫3RN在超临界二氧化碳中的溶解度:实验数据和模型(经验模型和热力学模型)
在这项研究中,在不同温度(308、318、328和338)K和最高压力34.0 MPa下,在有/无甲醇作为共溶剂的超临界CO 2中测量了蒽醌紫3RN(AV3RN)的溶解度。AV3RN的溶解度在0.047×10 -5至0.546×10 -5 摩尔分数的范围内变化,而其通过共溶剂的溶解度在0.44×10 -5至5.77×10 -5的范围内 摩尔分数。通过添加6%摩尔的甲醇作为助溶剂,计算得出的增加的溶解度因子在7.7-15.9范围内。对于这两个研究系统,AV3RN的溶解度都与几种基于密度的模型和状态方程(EoSs)相关,包括Peng-Robinson(PR)和Soave-Redlich-Kwong(SRK)。由Khansary等人开发的经验模型。以及二进制系统中的Garlapati–Madras模型以及Jouyban等人。三元系统中的模型显示出最高的符合实验数据。还表明,与SRK相比,PR模型在预测AV3RN的溶解度方面表现更好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号