当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biopharmaceutic In Vitro In Vivo Extrapolation (IVIV_E) Informed Physiologically-Based Pharmacokinetic Model of Ritonavir Norvir Tablet Absorption in Humans Under Fasted and Fed State Conditions.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.molpharmaceut.0c00043 Sumit Arora 1 , Amita Pansari 1 , Peter Kilford 1 , Masoud Jamei 1 , Iain Gardner 1 , David B Turner 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-19 , DOI: 10.1021/acs.molpharmaceut.0c00043 Sumit Arora 1 , Amita Pansari 1 , Peter Kilford 1 , Masoud Jamei 1 , Iain Gardner 1 , David B Turner 1
Affiliation
|
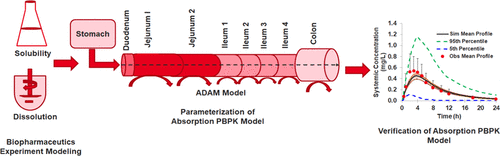
Ritonavir is a well-known CYP3A4 and CYP2D6 enzyme inhibitor, frequently used to assess the drug–drug interaction (DDI) liability of susceptible drugs. It is also used as a pharmacokinetic booster to increase exposure to CYP3A4 substrates. This study aimed to develop a mechanistic absorption and disposition model to describe exposure to ritonavir following oral dosing of the commercial amorphous solid dispersion tablet, Norvir, under fasted and fed conditions. A mechanistic description of ritonavir absorption from Norvir tablets may help to improve the design of DDI studies. Key parameters of amorphous ritonavir including free base solubility (solubility of the unbound, un-ionized species), bile micelle partition coefficients, formulation wetting/disintegration, and in vivo precipitation parameters were either obtained from the literature or estimated by modeling in vitro biopharmaceutic experiments. Based on variety of in vitro evidence, a main assumption of the model is that ritonavir does not form a crystalline precipitate while resident in the gastrointestinal tract. In the model, if simulated luminal concentration exceeds the amorphous solubility limit, then precipitation to an amorphous form is immediate. Simulated and observed Cmax and AUC0-t parameters were well captured (within 1.5-fold) for both fasted and fed states in healthy volunteers. By accounting for luminal fluid viscosity differences in the different prandial states (affecting drug diffusivity) as well as the effect of drug free fraction on gut wall permeation rates, it was possible to explain the negative food effect observed for Norvir tablets in humans. In summary, a biopharmaceutic in vitro in vivo extrapolation approach provides confidence in (verification of) key input parameters of the physiologically-based pharmacokinetic ritonavir model which resulted in successful simulation of observed plasma profiles.
中文翻译:

在禁食和进食状态下,利托那韦Norvir片剂在人体中的吸收基于生理学的生物药物体外动力学(IVIV_E)通知了基于生理的药代动力学模型。
Ritonavir是一种著名的CYP3A4和CYP2D6酶抑制剂,经常用于评估易感药物的药物相互作用。它也被用作药代动力学增强剂,以增加对CYP3A4底物的暴露。这项研究旨在建立一种机械吸收和处置模型,以描述在禁食和进食条件下口服无定形固体分散片Norvir后口服利托那韦的情况。从Norvir片剂吸收ritonavir的机制描述可能有助于改善DDI研究的设计。无定形利托那韦的关键参数包括游离碱溶解度(未结合,未电离物质的溶解度),胆胶束分配系数,制剂润湿/崩解和体内降水参数可以从文献中获得,也可以通过对体外生物制药实验进行建模来估算。基于各种体外证据,该模型的主要假设是利托那韦驻留在胃肠道中时不会形成结晶沉淀。在模型中,如果模拟的腔内浓度超过无定形溶解度极限,则立即沉淀为无定形形式。模拟和观察到的C max和AUC 0- t在健康志愿者中,禁食和进食状态的参数均被很好地捕获(1.5倍以内)。通过考虑不同饮食状态下的腔内流体粘度差异(影响药物扩散性)以及无药物组分对肠壁渗透率的影响,可以解释诺维片剂在人体中观察到的负面食物影响。总而言之,生物药物体外体内外推方法为基于生理学的药代动力学利托那韦模型的关键输入参数(的验证)提供了信心,从而成功地模拟了观察到的血浆分布。
更新日期:2020-07-06
中文翻译:

在禁食和进食状态下,利托那韦Norvir片剂在人体中的吸收基于生理学的生物药物体外动力学(IVIV_E)通知了基于生理的药代动力学模型。
Ritonavir是一种著名的CYP3A4和CYP2D6酶抑制剂,经常用于评估易感药物的药物相互作用。它也被用作药代动力学增强剂,以增加对CYP3A4底物的暴露。这项研究旨在建立一种机械吸收和处置模型,以描述在禁食和进食条件下口服无定形固体分散片Norvir后口服利托那韦的情况。从Norvir片剂吸收ritonavir的机制描述可能有助于改善DDI研究的设计。无定形利托那韦的关键参数包括游离碱溶解度(未结合,未电离物质的溶解度),胆胶束分配系数,制剂润湿/崩解和体内降水参数可以从文献中获得,也可以通过对体外生物制药实验进行建模来估算。基于各种体外证据,该模型的主要假设是利托那韦驻留在胃肠道中时不会形成结晶沉淀。在模型中,如果模拟的腔内浓度超过无定形溶解度极限,则立即沉淀为无定形形式。模拟和观察到的C max和AUC 0- t在健康志愿者中,禁食和进食状态的参数均被很好地捕获(1.5倍以内)。通过考虑不同饮食状态下的腔内流体粘度差异(影响药物扩散性)以及无药物组分对肠壁渗透率的影响,可以解释诺维片剂在人体中观察到的负面食物影响。总而言之,生物药物体外体内外推方法为基于生理学的药代动力学利托那韦模型的关键输入参数(的验证)提供了信心,从而成功地模拟了观察到的血浆分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号