当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting Pro-Tumoral Macrophages in Early Primary and Metastatic Breast Tumors with the CD206-Binding mUNO Peptide.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-18 , DOI: 10.1021/acs.molpharmaceut.0c00226 Anni Lepland 1 , Eliana K Asciutto 2 , Alessio Malfanti 3 , Lorena Simón-Gracia 1 , Valeria Sidorenko 1 , Maria J Vicent 3 , Tambet Teesalu 1, 4, 5 , Pablo Scodeller 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-05-18 , DOI: 10.1021/acs.molpharmaceut.0c00226 Anni Lepland 1 , Eliana K Asciutto 2 , Alessio Malfanti 3 , Lorena Simón-Gracia 1 , Valeria Sidorenko 1 , Maria J Vicent 3 , Tambet Teesalu 1, 4, 5 , Pablo Scodeller 1
Affiliation
|
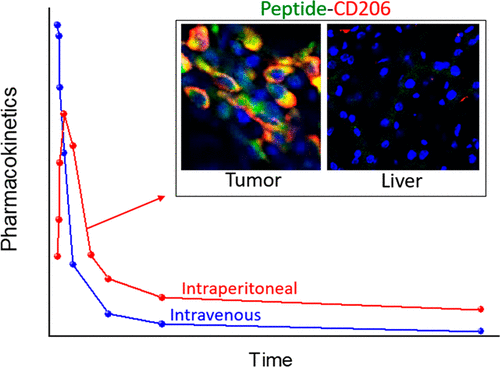
M2-like tumor-associated macrophages (M2 TAMs) play important roles in the resistance of tumors to immunotherapies. Selective depletion or reprogramming of M2 TAMs may sensitize the nonresponsive tumors for immune-mediated eradication. However, precision delivery of payloads to M2 TAMs, while sparing healthy tissues, has remained an unresolved challenge. Here, we studied the application of a short linear peptide (CSPGAK, “mUNO”) for the delivery of molecular and nanoscale cargoes in M2 TAMs in vitro and the relevance of the peptide for in vivo targeting of early-stage primary breast tumors and metastatic lung foci. First, we performed in silico modeling and found that mUNO interacts with mouse CD206 via a binding site between lectin domains CTLD1 and CTLD2, the same site previously demonstrated to be involved in mUNO binding to human CD206. Second, we showed that cultured M2 macrophages take up fluorescein-labeled (FAM) polymersomes conjugated with mUNO using the sulfhydryl group of its N-terminal cysteine. Pulse/chase studies of FAM-mUNO in M2 macrophages suggested that the peptide avoided lysosomal entrapment and escaped from early endosomes. Third, our in vivo studies with FAM-mUNO demonstrated that intraperitoneal administration results in better pharmacokinetics and higher blood bioavailability than can be achieved with intravenous administration. Intraperitoneal FAM-mUNO, but not FAM-control, showed a robust accumulation in M2-skewed macrophages in mouse models of early primary breast tumor and lung metastasis. This targeting was specific, as no uptake was observed in nonmalignant control organs, including the liver, or other cell types in the tumor, including M1 macrophages. Collectively, our studies support the application of the CD206-binding mUNO peptide for delivery of molecular and nanoscale cargoes to M2 macrophages and manifest the relevance of this mode of targeting primary and metastatic breast tumors.
中文翻译:

用CD206结合型mUNO肽靶向早期原发性和转移性乳腺肿瘤中的亲肿瘤巨噬细胞。
M2样肿瘤相关巨噬细胞(M2 TAM)在肿瘤对免疫疗法的抵抗中起重要作用。M2 TAM的选择性耗竭或重编程可能会使无反应的肿瘤对免疫介导的根除敏感。然而,有效载荷向M2 TAM的精确传递,同时又不损害健康组织,仍然是一个尚未解决的挑战。在这里,我们研究了短线性肽(CSPGAK,“ mUNO”)在M2 TAM中体外递送分子和纳米级货物的应用,以及该肽在体内靶向早期原发性乳腺肿瘤和转移性疾病的相关性肺病灶。首先,我们在计算机上表演建模并发现mUNO通过凝集素结构域CTLD1和CTLD2之间的结合位点与小鼠CD206相互作用,该位点先前已证明参与mUNO与人CD206的结合。其次,我们显示培养的M2巨噬细胞吸收了使用mUNO的N端半胱氨酸的巯基标记的荧光素标记(FAM)聚合物小体。在M2巨噬细胞中对FAM-mUNO进行脉冲/追踪研究表明,该肽避免了溶酶体的捕获,并逃脱了早期的内体。第三,我们的体内FAM-mUNO的研究表明,与静脉内给药相比,腹膜内给药具有更好的药代动力学和更高的血液生物利用度。在早期原发性乳腺肿瘤和肺转移的小鼠模型中,腹膜内FAM-mUNO(而非FAM对照)显示出M2偏向巨噬细胞中的强大积累。这种靶向是特异性的,因为在非恶性对照器官(包括肝脏)或肿瘤中其他细胞类型(包括M1巨噬细胞)中未观察到摄取。总的来说,我们的研究支持将CD206结合型mUNO肽用于将分子和纳米级货物递送至M2巨噬细胞,并证明这种靶向原发性和转移性乳腺肿瘤的模式具有相关性。
更新日期:2020-07-06
中文翻译:

用CD206结合型mUNO肽靶向早期原发性和转移性乳腺肿瘤中的亲肿瘤巨噬细胞。
M2样肿瘤相关巨噬细胞(M2 TAM)在肿瘤对免疫疗法的抵抗中起重要作用。M2 TAM的选择性耗竭或重编程可能会使无反应的肿瘤对免疫介导的根除敏感。然而,有效载荷向M2 TAM的精确传递,同时又不损害健康组织,仍然是一个尚未解决的挑战。在这里,我们研究了短线性肽(CSPGAK,“ mUNO”)在M2 TAM中体外递送分子和纳米级货物的应用,以及该肽在体内靶向早期原发性乳腺肿瘤和转移性疾病的相关性肺病灶。首先,我们在计算机上表演建模并发现mUNO通过凝集素结构域CTLD1和CTLD2之间的结合位点与小鼠CD206相互作用,该位点先前已证明参与mUNO与人CD206的结合。其次,我们显示培养的M2巨噬细胞吸收了使用mUNO的N端半胱氨酸的巯基标记的荧光素标记(FAM)聚合物小体。在M2巨噬细胞中对FAM-mUNO进行脉冲/追踪研究表明,该肽避免了溶酶体的捕获,并逃脱了早期的内体。第三,我们的体内FAM-mUNO的研究表明,与静脉内给药相比,腹膜内给药具有更好的药代动力学和更高的血液生物利用度。在早期原发性乳腺肿瘤和肺转移的小鼠模型中,腹膜内FAM-mUNO(而非FAM对照)显示出M2偏向巨噬细胞中的强大积累。这种靶向是特异性的,因为在非恶性对照器官(包括肝脏)或肿瘤中其他细胞类型(包括M1巨噬细胞)中未观察到摄取。总的来说,我们的研究支持将CD206结合型mUNO肽用于将分子和纳米级货物递送至M2巨噬细胞,并证明这种靶向原发性和转移性乳腺肿瘤的模式具有相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号