当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Urea-based imprinted polymer hosts with switchable anion preference
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-19 , DOI: 10.1021/jacs.0c00707 Sudhirkumar Shinde 1, 2 , Anil Incel 1 , Mona Mansour 1 , Gustaf D Olsson 3 , Ian A Nicholls 3 , Cem Esen 2 , Javier Urraca 2 , Börje Sellergren 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-19 , DOI: 10.1021/jacs.0c00707 Sudhirkumar Shinde 1, 2 , Anil Incel 1 , Mona Mansour 1 , Gustaf D Olsson 3 , Ian A Nicholls 3 , Cem Esen 2 , Javier Urraca 2 , Börje Sellergren 1, 2
Affiliation
|
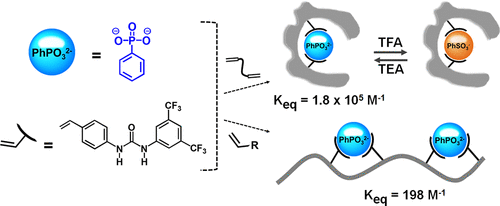
The design of artificial oxyanion receptors with switchable ion preference is a challenging goal in host–guest chemistry. We here report on molecularly imprinted polymers (MIPs) with an external phospho-sulpho switch driven by small molecule modifiers. The polymers were prepared by hydrogen bond-mediated imprinting of the mono- or dianions of phenyl phosphonic acid (PPA), phenyl sulfonic acid (PSA), and benzoic acid (BA) using N-3,5-bis-(trifluoromethyl)-phenyl-Ń-4-vinylphenyl urea (1) as the functional host monomer. The interaction mode between the functional monomer and the monoanions was elucidated by 1H NMR titrations and 1H–1H NMR NOESY supported by molecular dynamic simulation, which confirmed the presence of high-order complexes. PPA imprinted polymers bound PPA with an equilibrium constant Keq = 1.8 × 105 M–1 in acetonitrile (0.1% 1,2,2,6,6-pentamethylpiperidine) and inorganic HPO42– and SO42– with Keq = 2.9 × 103 M–1 and 4.5 × 103 M–1, respectively, in aqueous buffer. Moreover, the chromatographic retentivity of phosphonate versus sulfonate was shown to be completely switched on this polymer when changing from a basic to an acidic modifier. Mechanistic insights into this system were obtained from kinetic investigations and DSC-, MALDI-TOF-MS-, 1H NMR-studies of linear polymers prepared in the presence of template. The results suggest the formation of template induced 1–1 diad repeats in the polymer main chain shedding unique light on the relative contributions of configurational and conformational imprinting.
中文翻译:

具有可切换阴离子偏好的尿素基印迹聚合物主体
具有可切换离子偏好的人工氧阴离子受体的设计是主客体化学中的一个具有挑战性的目标。我们在此报告了具有由小分子改性剂驱动的外部磷硫开关的分子印迹聚合物 (MIP)。这些聚合物是通过氢键介导的苯基膦酸 (PPA)、苯基磺酸 (PSA) 和苯甲酸 (BA) 的单阴离子或双阴离子印迹制备的,使用 N-3,5-双-(三氟甲基)-苯基-Ń-4-乙烯基苯基脲 (1) 作为功能性主体单体。功能单体和单阴离子之间的相互作用模式通过 1H NMR 滴定和分子动力学模拟支持的 1H-1H NMR NOESY 阐明,证实了高阶配合物的存在。PPA 印迹聚合物以平衡常数 Keq = 1.8 × 105 M–1 在乙腈 (0.1% 1,2,2,6, 6-五甲基哌啶)和无机 HPO42– 和 SO42–,在水性缓冲液中,Keq 分别为 2.9 × 103 M–1 和 4.5 × 103 M–1。此外,当从碱性改性剂变为酸性改性剂时,膦酸盐与磺酸盐的色谱保留性显示完全切换到该聚合物上。通过动力学研究和 DSC-、MALDI-TOF-MS-、1H NMR-研究在模板存在下制备的线性聚合物获得对该系统的机理见解。结果表明在聚合物主链中模板诱导的 1-1 双单元重复的形成为构型和构象印记的相对贡献提供了独特的启示。当从碱性改性剂变为酸性改性剂时,膦酸盐与磺酸盐的色谱保留性显示完全切换到该聚合物上。通过动力学研究和 DSC-、MALDI-TOF-MS-、1H NMR-研究在模板存在下制备的线性聚合物获得对该系统的机理见解。结果表明在聚合物主链中模板诱导的 1-1 双单元重复的形成为构型和构象印记的相对贡献提供了独特的启示。当从碱性改性剂变为酸性改性剂时,膦酸盐与磺酸盐的色谱保留性显示完全切换到该聚合物上。通过动力学研究和 DSC-、MALDI-TOF-MS-、1H NMR-研究在模板存在下制备的线性聚合物获得对该系统的机理见解。结果表明在聚合物主链中模板诱导的 1-1 双单元重复的形成为构型和构象印记的相对贡献提供了独特的启示。
更新日期:2020-05-19
中文翻译:

具有可切换阴离子偏好的尿素基印迹聚合物主体
具有可切换离子偏好的人工氧阴离子受体的设计是主客体化学中的一个具有挑战性的目标。我们在此报告了具有由小分子改性剂驱动的外部磷硫开关的分子印迹聚合物 (MIP)。这些聚合物是通过氢键介导的苯基膦酸 (PPA)、苯基磺酸 (PSA) 和苯甲酸 (BA) 的单阴离子或双阴离子印迹制备的,使用 N-3,5-双-(三氟甲基)-苯基-Ń-4-乙烯基苯基脲 (1) 作为功能性主体单体。功能单体和单阴离子之间的相互作用模式通过 1H NMR 滴定和分子动力学模拟支持的 1H-1H NMR NOESY 阐明,证实了高阶配合物的存在。PPA 印迹聚合物以平衡常数 Keq = 1.8 × 105 M–1 在乙腈 (0.1% 1,2,2,6, 6-五甲基哌啶)和无机 HPO42– 和 SO42–,在水性缓冲液中,Keq 分别为 2.9 × 103 M–1 和 4.5 × 103 M–1。此外,当从碱性改性剂变为酸性改性剂时,膦酸盐与磺酸盐的色谱保留性显示完全切换到该聚合物上。通过动力学研究和 DSC-、MALDI-TOF-MS-、1H NMR-研究在模板存在下制备的线性聚合物获得对该系统的机理见解。结果表明在聚合物主链中模板诱导的 1-1 双单元重复的形成为构型和构象印记的相对贡献提供了独特的启示。当从碱性改性剂变为酸性改性剂时,膦酸盐与磺酸盐的色谱保留性显示完全切换到该聚合物上。通过动力学研究和 DSC-、MALDI-TOF-MS-、1H NMR-研究在模板存在下制备的线性聚合物获得对该系统的机理见解。结果表明在聚合物主链中模板诱导的 1-1 双单元重复的形成为构型和构象印记的相对贡献提供了独特的启示。当从碱性改性剂变为酸性改性剂时,膦酸盐与磺酸盐的色谱保留性显示完全切换到该聚合物上。通过动力学研究和 DSC-、MALDI-TOF-MS-、1H NMR-研究在模板存在下制备的线性聚合物获得对该系统的机理见解。结果表明在聚合物主链中模板诱导的 1-1 双单元重复的形成为构型和构象印记的相对贡献提供了独特的启示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号