当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism for Singlet Oxygen Production in Li-Ion and Metal–Air Batteries
ACS Energy Letters ( IF 22.0 ) Pub Date : 2020-05-19 , DOI: 10.1021/acsenergylett.0c00595 Gregory Houchins 1, 2 , Vikram Pande 3 , Venkatasubramanian Viswanathan 1, 2, 3
ACS Energy Letters ( IF 22.0 ) Pub Date : 2020-05-19 , DOI: 10.1021/acsenergylett.0c00595 Gregory Houchins 1, 2 , Vikram Pande 3 , Venkatasubramanian Viswanathan 1, 2, 3
Affiliation
|
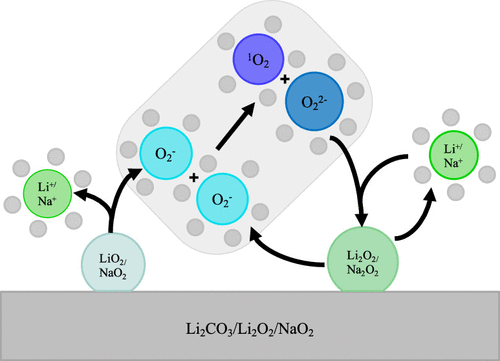
Singlet oxygen has emerged as a real mystery puzzling battery science, having been observed in Li–O2 and Na–O2 batteries, in conventional Li-ion batteries with NMC cathodes, and during the oxidation of Li2CO3. The formation of singlet oxygen has been directly linked to the degradation and catastrophic fade seen in these battery chemistries. While there are several proposed hypothesis for its origin, the exact mechanism for the formation of singlet oxygen remains unclear. In this Letter, we attempt to unify these findings by proposing a mechanism of singlet oxygen production in metal–air and Li-ion batteries. We show that a potential dependence of surface termination explains the onset potentials of singlet oxygen release, and in all considered cases the mechanism of singlet oxygen generation is through the chemical disproportionation of the uncoordinated superoxide anion in solution; therefore, the singlet oxygen yield is determined by the concentration of free superoxide versus alkali superoxide ion pairs in solution.
中文翻译:

锂离子和金属空气电池中单线态氧气产生的机理
单重态氧已经成为真正令人费解的电池科学,已经在Li-O 2和Na-O 2电池,具有NMC阴极的传统锂离子电池以及Li 2 CO 3的氧化过程中被观察到。。单线态氧的形成与这些电池化学中的降解和灾难性褪色直接相关。尽管有一些关于其起源的假说,但单线态氧形成的确切机理仍不清楚。在这封信中,我们试图通过提出一种在金属-空气和锂离子电池中产生单重态氧的机制来统一这些发现。我们表明表面终止的电位依赖性解释了单线态氧释放的开始电位,并且在所有考虑的情况下,单线态氧产生的机理是通过溶液中未配位的超氧阴离子的化学歧化;因此,单重态氧的产率取决于溶液中游离超氧化物对碱金属超氧化物离子对的浓度。
更新日期:2020-05-19
中文翻译:

锂离子和金属空气电池中单线态氧气产生的机理
单重态氧已经成为真正令人费解的电池科学,已经在Li-O 2和Na-O 2电池,具有NMC阴极的传统锂离子电池以及Li 2 CO 3的氧化过程中被观察到。。单线态氧的形成与这些电池化学中的降解和灾难性褪色直接相关。尽管有一些关于其起源的假说,但单线态氧形成的确切机理仍不清楚。在这封信中,我们试图通过提出一种在金属-空气和锂离子电池中产生单重态氧的机制来统一这些发现。我们表明表面终止的电位依赖性解释了单线态氧释放的开始电位,并且在所有考虑的情况下,单线态氧产生的机理是通过溶液中未配位的超氧阴离子的化学歧化;因此,单重态氧的产率取决于溶液中游离超氧化物对碱金属超氧化物离子对的浓度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号