Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2020-04-24 , DOI: 10.1111/imcb.12332 Adrian Liston 1 , Carly E Whyte 1
|
The microbiome is increasingly recognized for its ability to modulate human health. Colonization with gut symbionts induces Foxp3‐expressing regulatory T cells (Tregs) and expands their local numbers, a critical step in the suppression of intestinal inflammation and maintaining gut homeostasis. The molecular mechanism by which the microbiome interacts with peripherally induced Treg (pTreg) is likely complex and multifactorial; however, part of the effect is mediated via the release of microbial fermentation products, such as butyrate and other short‐chain fatty acids.1-3 In a string of recent studies, a role for host bile acids has also been shown to induce pTreg generation, with this function dependent on commensal colonization.4-6 In the recent issue of Nature, Rudensky and colleagues dissect the molecular fermentation pathway where the gut microbiome converts an endogenous bile acid into an immunologically active bile acid with the capacity to induce pTreg in the gut.4
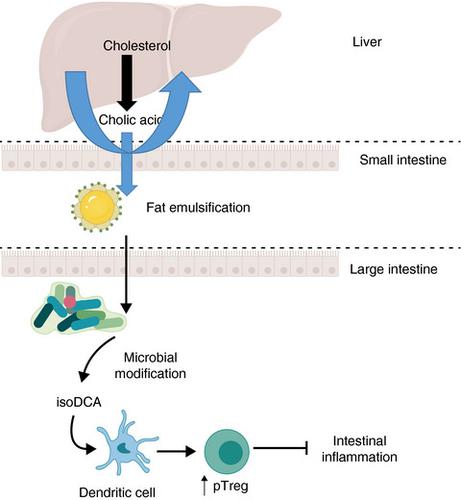
Primary bile acids are cholesterol‐derived molecules that are produced in the liver and secreted into the duodenum following a meal. Because of their amphipathic nature, these molecules help to dissolve dietary lipids, as well as having key roles in glucose homeostasis and antimicrobial defense. Although most bile acids are reabsorbed in the small intestine, approximately 5% of primary bile acids transit to the colon. This bile acid waste can be metabolized by commensal gut flora, with the modified structures of the secondary bile acids altering signaling potential. Key modifications include deconjugation by bacterial bile salt hydrolases. Despite research into bile acids and their metabolic effects being a relatively mature field, it is not well understood how these molecules interact with the adaptive immune system.
To address this issue, Campbell et al.4 set out to discover secondary bile acids capable of modulating pTreg generation in mice. Through an in vitro screen of major types of bile acids found in mice and humans on Treg differentiation, 3β‐hydroxydeoxycholic acid (isoDCA) and ω‐muricholic acid were identified as capable of enhancing Treg generation without impacting the conventional Th17 population. The Treg‐promoting ability of isoDCA, one of the most abundant bile acids found in humans, was dependent on the presence of dendritic cells in the culture. Treatment of dendritic cells in vitro with isoDCA reduced the production of inflammatory cytokines tumor necrosis factor and interleukin‐6, as well as reducing T‐cell priming, demonstrating an immunosuppressive role for this bile acid. Transcriptome analysis of dendritic cells treated with isoDCA demonstrated that isoDCA reduced the expression of antigen‐processing and antigen‐presentation genes, potentially disturbing cognate interactions with T cells.
Many biological effects of bile acids are exerted through binding to the Farnesoid X receptor (FXR). Comparison of the transcriptome of FXR‐deficient dendritic cells identified a mirrored transcriptional profile to isoDCA treatment, suggesting the isoDCA acts as a natural antagonist of FXR signaling. The Treg expansion effect observed for isoDCA was likewise replicated by FXR deficiency, with knockout dendritic cells demonstrating the same in vitro escalation of Treg induction even in the absence of isoDCA. In vivo, mice lacking FXR expression in the myeloid compartment (Csf1rcre Nr1h4fl/fl mice) displayed increased numbers of Foxp3+ Rorγt+ cells in the large intestine lamina propria, with this population predominantly representing pTreg induced in response to microbial antigens. Together, these data indicate that signaling through FXR in myeloid cells can inhibit pTreg generation. While FXR antagonism may account for much of the biological effect of isoDCA, however, isoDCA‐induced transcriptional changes are still produced in FXR‐deficient dendritic cells, indicating a still‐hidden additional layer of mechanism.
Campbell et al.4 formally linked microbial fermentation of isoDCA to colonic Treg expansion through the engineering of a minimal bacterial consortia. IsoDCA is generated from the primary bile acid cholic acid, with two structural changes required: cleavage of the 7α‐hydroxyl group and epimerization of the 3α‐hydroxyl group. To generate isoDCA in vivo, hydroxysteroid dehydrogenase was inserted into three independent Bacteroides species, to enable the epimerization of the 3α‐hydroxyl group of cholic acid. These bacteria were inoculated into mice along with a commensal capable of 7α‐dehydroxylation, providing the required microbial enzymes for de novo isoDCA generation. Colonization of germ‐free mice with this consortium increased colonic Treg cells to a greater extent than the control strains, engineered with a catalytically dead hydroxysteroid dehydrogenase (Figure 1). The effect was also observed, to a lower degree, in the control strains, again demonstrating that there is more complexity in the situation than a single fermentation pathway.

The effect of isoDCA on dendritic cells in this study is in contrast to recent work of T‐cell‐modulating bile acids, which potentiate Treg generation in a cell‐intrinsic manner.5, 6 This suggests that bile acids have diverse effects on the adaptive immune system and affect multiple cell types, opening the door for further research on how microbial metabolites can influence immune responses. Further complexity is added to the system through the effect of diet, which can influence the composition of both bile acids and the microbiota. A recent study in Nature6 demonstrated that mice fed a nutrient‐poor diet showed reduced presence of bile acids and subsequent reduction in pTreg generation. Mice fed a minimal diet were susceptible to intestinal inflammation, an effect which could be blocked through supplementation with select bile acids. Gastrointestinal health is therefore shaped through a complex interplay between bile acids, diet, the microbiome and our adaptive immune systems. The huge variation in these factors between individuals may contribute to the diversity we see in intestinal health, and provide attractive therapeutic strategies for intestinal disease.
中文翻译:

胆汁酸介导微生物组和免疫系统之间的信号传递
微生物组因其调节人类健康的能力而受到越来越多的认可。肠道共生菌定植可诱导表达Foxp3的调节性T细胞(Treg)并扩大其局部数量,这是抑制肠道炎症和维持肠道稳态的关键步骤。微生物组与周围诱导的Treg(pTreg)相互作用的分子机制可能是复杂的且是多因素的。但是,部分影响是通过释放微生物发酵产物(例如丁酸盐和其他短链脂肪酸)来介导的。1-3在一系列最新研究中,宿主胆汁酸的作用也已显示出诱导pTreg生成的功能,该功能取决于共生定植。4-6在最近的《自然》杂志上,Rudensky及其同事剖析了分子发酵途径,其中肠道微生物组将内源性胆汁酸转化为具有免疫活性的胆汁酸,并具有在肠道中诱导pTreg的能力。4
初级胆汁酸是胆固醇产生的分子,在肝脏中产生并在进餐后分泌到十二指肠。由于它们的两亲性质,这些分子有助于溶解饮食中的脂质,并且在葡萄糖稳态和抗菌防御中起关键作用。尽管大多数胆汁酸在小肠中被重吸收,但大约5%的初级胆汁酸转移到结肠。这种胆汁酸废物可以通过共生肠道菌群代谢,而次级胆汁酸的修饰结构改变了信号传导潜能。关键的修饰包括通过细菌胆汁盐水解酶的结合。尽管对胆汁酸及其代谢作用的研究是一个相对成熟的领域,但人们对这些分子如何与适应性免疫系统相互作用尚不甚了解。
为了解决这个问题,Campbell等人。4着手发现能够调节小鼠pTreg生成的仲胆汁酸。通过体外筛选小鼠和人类中Treg分化过程中发现的主要胆汁酸类型,确定3β-羟基脱氧胆酸(isoDCA)和ω-多酚酸能够增强Treg生成而不会影响常规Th17群体。isoDCA是人类发现的最丰富的胆汁酸之一,其Treg促进能力取决于培养物中树突状细胞的存在。体外治疗树突状细胞使用isoDCA可以减少炎性细胞因子肿瘤坏死因子和白细胞介素6的产生,并减少T细胞启动,证明该胆汁酸具有免疫抑制作用。用isoDCA处理的树突状细胞的转录组分析表明,isoDCA降低了抗原加工和抗原呈递基因的表达,可能干扰与T细胞的同源相互作用。
胆汁酸的许多生物学作用是通过与Farnesoid X受体(FXR)结合而产生的。缺乏FXR的树突状细胞的转录组的比较确定了镜像到isoDCA处理的转录谱,表明isoDCA是FXR信号传导的天然拮抗剂。FXR缺乏也复制了对isoDCA观察到的Treg扩增作用,即使在没有isoDCA的情况下,敲除的树突状细胞也显示出相同的Treg诱导体外扩增。在体内,在髓样区室中缺乏FXR表达的小鼠(Csf1r cre Nr1h4 fl / fl小鼠)显示出Foxp3 + Rorγt +大肠固有层中的细胞,该群体主要代表响应微生物抗原而诱导的pTreg。总之,这些数据表明在髓样细胞中通过FXR进行的信号传导可以抑制pTreg的产生。尽管FXR拮抗作用可能是isoDCA的大部分生物学效应,但是,在FXR缺陷的树突状细胞中,仍会产生isoDCA诱导的转录变化,这表明机制仍被隐藏。
坎贝尔等。4通过最小细菌菌群的改造,将isoDCA的微生物发酵与结肠Treg扩展形式正式联系起来。IsoDCA是由胆汁酸胆酸生成的,需要进行两个结构上的改变:7α-羟基的裂解和3α-羟基的差向异构化。为了在体内产生isoDCA ,将羟基类固醇脱氢酶插入三个独立的拟杆菌属物种中,以使胆酸的3α-羟基能够差向异构化。将这些细菌与能够进行7α-去羟基化的共生菌一起接种到小鼠体内,为新生提供所需的微生物酶。isoDCA生成。用这个财团对无菌小鼠进行定殖比对照菌株(用催化性死亡的羟类固醇脱氢酶改造的菌株)在更大程度上增加了结肠Treg细胞的数量(图1)。在对照菌株中也观察到了较低程度的效果,再次表明,与单一发酵途径相比,情况更为复杂。

在这项研究中,isoDCA对树突状细胞的作用与T细胞调节胆汁酸的最新研究形成了鲜明对比,后者以细胞内在的方式增强了Treg的产生。5,6这表明胆汁酸对适应性免疫系统具有多种作用,并影响多种细胞类型,为进一步研究微生物代谢物如何影响免疫反应打开了大门。通过饮食的影响,系统进一步增加了复杂性,这可能会影响胆汁酸和微生物群的组成。Nature 6的最新研究证明了饮食营养不良的小鼠胆汁酸的存在减少,随后pTreg产生减少。进食少量饮食的小鼠容易受到肠道炎症的影响,可通过补充精选的胆汁酸来阻止这种作用。因此,通过胆汁酸,饮食,微生物组和我们的适应性免疫系统之间的复杂相互作用,可以影响胃肠道的健康。这些因素之间个体之间的巨大差异可能有助于我们在肠道健康方面看到多样性,并为肠道疾病提供有吸引力的治疗策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号