Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2020-04-27 , DOI: 10.1111/imcb.12333 Amy A Baxter 1 , Ivan KH Poon 1
|
The rapid removal of apoptotic cells by phagocytes is a crucial homeostatic process that prevents dying cell accumulation and leakage of proinflammatory factors from dead cells into the extracellular environment. Over the past 20 years, the concept of apoptotic cell sensing by phagocytes via the release or exposure of “find‐me,” “keep‐out,” “eat‐me” and “don’t eat‐me” signals from apoptotic cells has become central to our understanding of how apoptotic cells facilitate their clearance while promoting an anti‐inflammatory response.1-5 In addition to this repertoire of signals, a novel form of intercellular communication by which apoptotic cells can regulate immune homeostasis has been reported. In a recent study by Medina et al.6 published in the journal Nature, certain cellular metabolites, termed “good‐bye” signals, were shown to be selectively released by apoptotic cells and could modulate the gene expression of healthy neighboring cells to regulate inflammation. The findings from this study reflect a continuing departure from the dogma that apoptotic “corpses” are merely cellular waste requiring disposal.
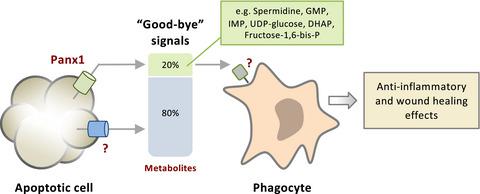
Work from the Center for Cell Clearance, at the University of Virginia, has defined the apoptotic cell “metabolite secretome” through a nonbiased metabolomics screen of apoptotic cell supernatants. Intriguingly, a selection of metabolites released by apoptotic cells was both caspase dependent and selective, that is, they appeared to be secreted despite other metabolites being retained within the dying cells. Notably, six metabolites (i.e. spermidine, adenosine triphosphate, adenosine monophosphate, guanosine monophosphate, creatine and glycerol‐3‐P) were found to be released by a range of cell types undergoing apoptosis. Continuing on from the authors previous work identifying caspase‐activated Pannexin 1 (Panx1) as a key membrane channel facilitating the release of adenosine triphosphate to function as a “find‐me” signal,7 20% of metabolites secreted by apoptotic cells (including the six aforesaid metabolites) were found to be dependent on Panx1 (Figure 1). The fact that only one‐fifth of apoptotic cell‐derived metabolites were shown to be released through Panx1 is of interest, as most metabolites are small (approximately 50–1500 Da8) and Panx1 has been suggested to facilitate the release of molecules that are up to 1000 Da.9 These observations suggest that additional mechanisms must exist to aid the release of the remaining 80% of metabolites from apoptotic cells, possibly via other membrane channels with activity regulated (directly or indirectly) by caspase activation (Figure 1). Caspase‐activated Panx1 has been described to regulate a broad range of processes during apoptosis, including the recruitment of phagocytes toward dying cells though the release of “find‐me” signals adenosine triphosphate and uridine triphosphate,7 the immune response through the release of “calm‐down” signal adenosine monophosphate10 and other metabolites (as will be detailed later), the disassembly of apoptotic cells into fragments known as apoptotic bodies to aid cell clearance11, 12 and NLRP3 inflammasome assembly.13 Precisely how these Panx1‐regulated processes intertwine to modulate cell clearance and immunity remains to be fully defined.

The release of cytokines and small extracellular vesicles (EVs) from apoptotic cells has been shown to facilitate communication with neighboring cells, in particular in the context of modulating inflammation and immunity.14-16 To this end, Medina and colleagues identified that several gene programs including those associated with regulating inflammation, tissue repair and metabolism were upregulated in phagocytes exposed to apoptotic cell supernatants. Importantly, for several of these programs, upregulation occurred specifically when supernatants were derived from apoptotic cells with active Panx1 channels (Figure 1). Using a murine model of thymocyte apoptosis the authors found that in vivo Panx1‐dependent metabolite release upregulated several genes in thymic phagocytic myeloid cells, in particular genes associated with anti‐inflammatory processes. These observations raise the compelling question of how, under physiological settings, other extracellular factors derived from apoptotic cells might influence phagocytes in conjunction with secreted metabolites. Presumably, the release of metabolites by an apoptotic cell into the local extracellular environment would occur amidst a milieu of proteins and EVs that could affect gene expression and/or function of neighboring cells. Indeed, a previous study by Jansen and colleagues in atherosclerotic mice reported that EV‐mediated transfer of microRNA from apoptotic to healthy endothelial cells downregulated expression of inflammatory markers in target cells.17 Likewise, EVs derived from apoptotic glioblastoma cells have been shown to mediate transfer of splicing factor RNA Binding Motif Protein 11 to neighboring cells, resulting in altered protein expression and promoting a more aggressive phenotype.18 Hence, how these different components could act in concert, as well as the kinetics of metabolite release in relation to EV and cytokine release is of significant interest. Medina and colleagues leave us with the intriguing concept that different cell types undergoing apoptosis can release unique combinations of metabolites, suggesting that changes in the metabolic secretome may exert different physiological and functional effects on neighboring cells. How this translates to regulation of inflammatory processes in the context of homeostasis, and moreover disease, remains largely unexplored.
Following identification that the Panx1‐dependent apoptotic cell metabolite secretome contains factors that could influence the gene expression of phagocytes, Medina and colleagues extended their studies to address in what pathological contexts this might operate. To this end, the effects of directly administering an exogenous metabolite mixture (coined as “MeMix”,3 containing Panx1‐dependent metabolites spermidine, guanosine monophosphate and inosine monophosphate) with an anti‐inflammatory signature in two murine inflammatory models were examined. In the first, arthritic mice were treated by intraperitoneal administration with MeMix,3 and displayed significant reductions in inflammation and other arthritic phenotypes. In the second, a lung transplant rejection model, mice transplanted with minor antigen‐mismatched lung allografts were intraperitoneally administered with MeMix3 post‐transplant. Remarkably, MeMix3‐treated mice displayed reduced organ rejection. Together, these findings provide proof‐of‐concept evidence that metabolites, in particular those secreted during apoptosis, can exhibit anti‐inflammatory properties in disease settings. It should be noted that the application of metabolites for therapeutic purposes is an area of growing interest, with recently reported examples including the use of microbiota‐derived metabolites in the maintenance of intestinal homeostasis,19 and the treatment of retinal neurodegeneration with oxidative phosphorylation and tricarboxylic acid cycle metabolites.20 Whether MeMix3 or other combinations of metabolites found in the apoptotic cell metabolite secretome could be developed as new therapeutics for inflammatory diseases would certainly be of interest in future studies. Furthermore, it is also of importance to elucidate the molecular factors on phagocytes that could meditate the detection of apoptotic cell‐derived metabolites (Figure 1), such as the potential role of solute carrier transporters,21 transient receptor potential cation channel subfamily V member 1 (TRPV1) channels22 and N‐methyl‐D‐aspartate receptors23 in sensing apoptotic cells.
Saying good‐bye can be hard, and dying cells have surely developed a remarkable way to part with their neighboring cells. The elegant and thought‐provoking work by Medina and colleagues has opened a new area of research, with many questions remaining to be explored.
中文翻译:

凋亡细胞分泌代谢产物来调节免疫稳态
吞噬细胞快速清除凋亡细胞是至关重要的体内平衡过程,可防止垂死的细胞积累和促炎因子从死细胞渗入细胞外环境。在过去的20年中,吞噬细胞通过释放或暴露凋亡细胞的“ find-me”,“ keep-out”,“ eat-me”和“ do n't me-me”信号来感知凋亡细胞的概念已经成为我们了解凋亡细胞如何促进其清除并促进抗炎反应的关键。1-5除了这些信号外,还报道了一种新型的细胞间通讯形式,凋亡细胞可以通过这种形式调节免疫稳态。在Medina等人的最新研究中。6在杂志上发表自然,某些细胞代谢产物,被称为“再见”信号,被凋亡细胞选择性释放,并可以调节健康邻近细胞的基因表达来调节炎症。这项研究的发现反映出,细胞凋亡的“尸体”仅仅是细胞废物,需要处理,这与教条的不断偏离。
弗吉尼亚大学细胞清除中心的工作通过凋亡细胞上清液的无偏代谢组学筛选确定了凋亡细胞“代谢物分泌物组”。有趣的是,凋亡细胞释放的代谢物的选择既是胱天蛋白酶依赖性的又是选择性的,也就是说,尽管其他代谢物保留在垂死的细胞中,但它们似乎是分泌的。值得注意的是,发现许多细胞凋亡类型的细胞释放了六种代谢物(即亚精胺,三磷酸腺苷,单磷酸腺苷,单磷酸鸟苷,肌酸和甘油3-P)。继续作者先前的工作,确定胱天蛋白酶激活的Pannexin 1(Panx1)是关键膜通道,可促进三磷酸腺苷的释放,起到“寻找我”的作用,7发现凋亡细胞分泌的代谢物(包括上述6种代谢物)的20%依赖于Panx1(图1)。有趣的是,只有五分之一的凋亡细胞代谢产物通过Panx1释放,这一事实很有意义,因为大多数代谢物很小(约50-1500 Da 8),而Panx1被认为可以促进释放高达1000 Da 9这些观察结果表明,必须存在其他机制来帮助凋亡细胞释放剩余的80%代谢物,可能是通过其他膜通道释放活性,而其活性(直接或间接)受caspase激活调节(图1)。胱天蛋白酶活化的Panx1已经描述调节范围广泛的凋亡过程中处理,包括吞噬细胞的朝向濒死细胞招募虽然发布的“找到我”信号腺苷三磷酸和尿苷三磷酸,7通过的“释放的免疫应答“冷静下来”信号表示单磷酸腺苷10和其他代谢物(将在后面详细介绍),将凋亡细胞分解为称为凋亡小体的碎片,以帮助清除细胞11、12和NLRP3炎症小体装配体。13确切地说,这些Panx1调控的过程如何交织以调节细胞清除率和免疫力仍有待完全确定。

已经显示出从凋亡细胞释放细胞因子和小的细胞外小泡(EVs)促进与邻近细胞的通讯,特别是在调节炎症和免疫力的情况下。14-16为此,Medina及其同事发现,暴露于凋亡细胞上清液的吞噬细胞中的几个基因程序(包括与调节炎症,组织修复和代谢相关的那些程序)均被上调。重要的是,对于其中几个程序,当上清液来自具有主动Panx1通道的凋亡细胞时,上调特别发生(图1)。用胸腺细胞的小鼠模型细胞凋亡的研究人员发现,在 体内Panx1依赖性代谢产物的释放上调了胸腺吞噬性髓细胞中的几个基因,特别是与抗炎过程相关的基因。这些发现提出了一个令人信服的问题,即在生理环境下,凋亡细胞衍生的其他细胞外因子如何与分泌的代谢物一起影响吞噬细胞。据推测,凋亡细胞将代谢物释放到局部细胞外环境中会发生在可能影响邻近细胞基因表达和/或功能的蛋白质和电动汽车的环境中。确实,Jansen及其同事先前在动脉粥样硬化小鼠中进行的一项研究报告说,EV介导的microRNA从凋亡细胞向健康内皮细胞的转移下调了靶细胞中炎性标志物的表达。17同样,从凋亡的胶质母细胞瘤细胞衍生的EV已显示出介导剪接因子RNA结合基序蛋白11向邻近细胞的转移,从而导致蛋白表达改变并促进更具攻击性的表型。18因此,这些不同的成分如何协同作用,以及与EV和细胞因子释放相关的代谢物释放动力学非常重要。Medina及其同事给我们留下了一个有趣的概念,即经历凋亡的不同细胞类型可以释放独特的代谢产物组合,这表明代谢分泌组的变化可能对邻近细胞产生不同的生理和功能作用。在稳态和疾病的背景下,这如何转化为炎症过程的调控,目前尚待探索。
鉴定出Panx1依赖性凋亡细胞代谢物分泌组包含可能影响吞噬细胞基因表达的因素后,Medina及其同事扩展了研究范围,以解决其可能在何种病理情况下起作用。为此,直接施用外源性代谢物的混合物的影响(精压为“MeMix”,3两种鼠炎症模型与抗炎签名含有Panx1依赖性代谢物亚精胺,鸟苷酸和肌苷单磷酸)进行了研究。首先,通过腹膜内给予MeMix,3治疗关节炎小鼠并显示出炎症和其他关节炎表型的显着减少。在第二种肺移植排斥模型中,将移植了少量抗原不匹配的肺同种异体移植的小鼠腹膜内给予MeMix 3移植后。值得注意的是,用MeMix 3处理的小鼠显示出减少的器官排斥。在一起,这些发现提供了概念证明,即代谢物,特别是凋亡过程中分泌的代谢物,在疾病环境中可以表现出抗炎特性。应当指出的是,将代谢物用于治疗目的已成为人们日益关注的领域,最近报道的例子包括微生物来源的代谢物在维持肠道动态平衡中的应用,19氧化磷酸化和三羧酸循环代谢物治疗视网膜神经变性。20是否可以开发出MeMix 3或凋亡细胞代谢物分泌物组中发现的其他代谢物组合作为炎症性疾病的新疗法,将在未来的研究中引起人们的兴趣。此外,阐明吞噬细胞上可能有助于检测凋亡性细胞衍生代谢产物的分子因素(图1)也很重要,例如溶质载体转运蛋白的潜在作用,21个瞬时受体电位阳离子通道亚家族V成员1 (TRPV1)通道22和N-甲基-D-天门冬氨酸受体23 在检测凋亡细胞中。
说再见可能很困难,垂死的细胞肯定已经发展出一种出色的方式来与周围的细胞分开。麦迪纳(Medina)及其同事的优雅而发人深省的工作开辟了一个新的研究领域,许多问题尚待探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号