当前位置:
X-MOL 学术
›
Immunol. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adipose-resident myeloid-derived suppressor cells modulate immune cell homeostasis in healthy mice.
Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2020-05-11 , DOI: 10.1111/imcb.12360 Katlin B Stivers 1, 2 , Paula M Chilton 1, 2 , Jason E Beare 1 , Jacob R Dale 1 , Pascale Alard 2 , Amanda J LeBlanc 1, 3 , James B Hoying 1, 3
Immunology and Cell Biology ( IF 3.2 ) Pub Date : 2020-05-11 , DOI: 10.1111/imcb.12360 Katlin B Stivers 1, 2 , Paula M Chilton 1, 2 , Jason E Beare 1 , Jacob R Dale 1 , Pascale Alard 2 , Amanda J LeBlanc 1, 3 , James B Hoying 1, 3
Affiliation
|
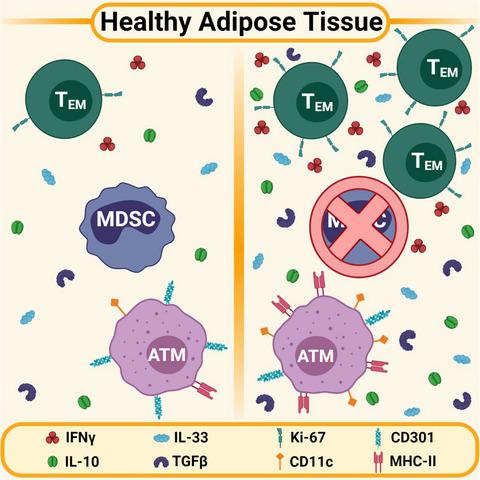
The metabolically dynamic nature of healthy adipose places this tissue under regular inflammatory stress. A network of adipose‐resident anti‐inflammatory immune cells modulates and resolves this endogenous inflammation. Previous work in our laboratory identified a CD11b+Gr1+ subset of these immunosuppressive adipose stromal cells in healthy mice. Myeloid‐derived suppressor cells (MDSCs), typically associated with cancer and chronic inflammation, have a similar surface marker phenotype and accumulate in adipose of high‐fat diet‐fed mice. Given the routine inflammatory stresses on healthy adipose and the suppressive nature of the tissue‐resident immune cells, we hypothesized that these CD11b+Gr1+ cells were a genuine population of MDSCs involved in regulating tissue homeostasis. Flow cytometric analysis of these cells found that they were CD11b+CD301− Ly6C+Ly6G+/− and did not express traditional macrophage markers. Moreover, in vitro functional assays demonstrated that these cells suppressed αCD3/αCD28‐induced T‐cell proliferation, solidifying their identity as bona fide adipose‐resident MDSCs. Systemic MDSC depletion altered adipose immune cell dynamics in otherwise healthy mice, increasing the number of CD4+ effector memory T cells and modifying the surface markers expressed by adipose‐resident macrophages. In addition, transcription of various immunomodulatory cytokines was clearly dysregulated in the adipose of MDSC‐depleted animals compared with controls. Altogether, our findings indicate that there is a population of bona fide MDSCs in the adipose of otherwise healthy mice that actively contribute to the health and immune homeostasis of this tissue.
中文翻译:

脂肪驻留的髓源性抑制细胞调节健康小鼠的免疫细胞稳态。
健康脂肪的代谢动态特性使该组织处于常规炎症应激之下。脂肪驻留抗炎免疫细胞网络调节和解决这种内源性炎症。我们实验室之前的工作在健康小鼠中鉴定了这些免疫抑制脂肪基质细胞的 CD11b + Gr1 +子集。髓源性抑制细胞 (MDSCs) 通常与癌症和慢性炎症相关,具有相似的表面标志物表型,并在高脂肪饮食喂养的小鼠的脂肪中积累。鉴于健康脂肪的常规炎症应激和组织驻留免疫细胞的抑制性质,我们假设这些 CD11b + Gr1 +细胞是参与调节组织稳态的真正的 MDSC 群体。这些细胞的流式细胞术分析发现它们是 CD11b + CD301 − Ly6C + Ly6G +/-并且不表达传统的巨噬细胞标志物。此外,体外功能分析表明,这些细胞抑制了 αCD3/αCD28 诱导的 T 细胞增殖,巩固了它们作为真正的脂肪驻留MDSC 的身份。全身性 MDSC 耗竭改变了健康小鼠的脂肪免疫细胞动力学,增加了 CD4 +的数量效应记忆 T 细胞和修饰脂肪驻留巨噬细胞表达的表面标志物。此外,与对照组相比,MDSC 耗竭动物的脂肪中各种免疫调节细胞因子的转录明显失调。总而言之,我们的研究结果表明,在其他方面健康的小鼠的脂肪中存在一群真正的MDSC,它们积极促进该组织的健康和免疫稳态。
更新日期:2020-05-11
中文翻译:

脂肪驻留的髓源性抑制细胞调节健康小鼠的免疫细胞稳态。
健康脂肪的代谢动态特性使该组织处于常规炎症应激之下。脂肪驻留抗炎免疫细胞网络调节和解决这种内源性炎症。我们实验室之前的工作在健康小鼠中鉴定了这些免疫抑制脂肪基质细胞的 CD11b + Gr1 +子集。髓源性抑制细胞 (MDSCs) 通常与癌症和慢性炎症相关,具有相似的表面标志物表型,并在高脂肪饮食喂养的小鼠的脂肪中积累。鉴于健康脂肪的常规炎症应激和组织驻留免疫细胞的抑制性质,我们假设这些 CD11b + Gr1 +细胞是参与调节组织稳态的真正的 MDSC 群体。这些细胞的流式细胞术分析发现它们是 CD11b + CD301 − Ly6C + Ly6G +/-并且不表达传统的巨噬细胞标志物。此外,体外功能分析表明,这些细胞抑制了 αCD3/αCD28 诱导的 T 细胞增殖,巩固了它们作为真正的脂肪驻留MDSC 的身份。全身性 MDSC 耗竭改变了健康小鼠的脂肪免疫细胞动力学,增加了 CD4 +的数量效应记忆 T 细胞和修饰脂肪驻留巨噬细胞表达的表面标志物。此外,与对照组相比,MDSC 耗竭动物的脂肪中各种免疫调节细胞因子的转录明显失调。总而言之,我们的研究结果表明,在其他方面健康的小鼠的脂肪中存在一群真正的MDSC,它们积极促进该组织的健康和免疫稳态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号