当前位置:
X-MOL 学术
›
Clin. Transl. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-dimensional analyses reveal a distinct role of T-cell subsets in the immune microenvironment of gastric cancer.
Clinical & Translational Immunology ( IF 4.6 ) Pub Date : 2020-05-05 , DOI: 10.1002/cti2.1127 Minyu Wang 1, 2, 3, 4 , Yu-Kuan Huang 1, 2, 3 , Joseph Ch Kong 2, 3 , Yu Sun 2 , Daniela G Tantalo 4 , Han Xian Aw Yeang 4 , Le Ying 5 , Feng Yan 6 , Dakang Xu 7 , Heloise Halse 4 , Natasha Di Costanzo 1 , Ian R Gordon 8 , Catherine Mitchell 9 , Laura K Mackay 10 , Rita A Busuttil 1, 2, 3 , Paul J Neeson 2, 4, 11 , Alex Boussioutas 1, 2, 3
Clinical & Translational Immunology ( IF 4.6 ) Pub Date : 2020-05-05 , DOI: 10.1002/cti2.1127 Minyu Wang 1, 2, 3, 4 , Yu-Kuan Huang 1, 2, 3 , Joseph Ch Kong 2, 3 , Yu Sun 2 , Daniela G Tantalo 4 , Han Xian Aw Yeang 4 , Le Ying 5 , Feng Yan 6 , Dakang Xu 7 , Heloise Halse 4 , Natasha Di Costanzo 1 , Ian R Gordon 8 , Catherine Mitchell 9 , Laura K Mackay 10 , Rita A Busuttil 1, 2, 3 , Paul J Neeson 2, 4, 11 , Alex Boussioutas 1, 2, 3
Affiliation
|
Objectives
To facilitate disease prognosis and improve precise immunotherapy of gastric cancer (GC) patients, a comprehensive study integrating immune cellular and molecular analyses on tumor tissues and peripheral blood was performed.
Methods
The association of GC patients' outcomes and the immune context of their tumors was explored using multiplex immunohistochemistry (mIHC) and transcriptome profiling. Potential immune dysfunction mechanism/s in the tumors on the systemic level was further examined using mass cytometry (CyTOF) in complementary peripheral blood from selected patients. GC cohorts with mIHC and gene expression profiling data were also used as validation cohorts.
Results
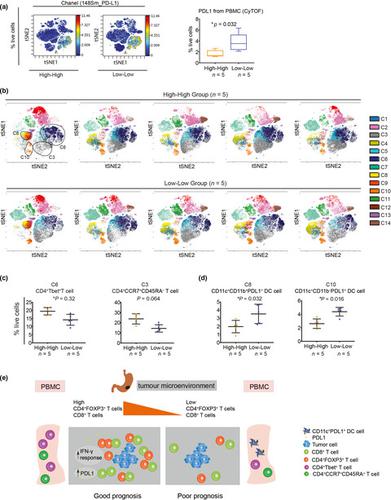
Increased CD4+FOXP3+ T-cell density in the GC tumor correlated with prolonged survival. Interestingly, CD4+FOXP3+ T cells had a close interaction with CD8+ T cells rather than tumor cells. High densities of CD4+FOXP3+ T cells and CD8+ T cells (High-High) independently predicted prolonged patient survival. Furthermore, the interferon-gamma (IFN-γ) gene signature and PDL1 expression were up-regulated in this group. Importantly, a subgroup of genomically stable (GS) tumors and tumors with chromosomal instability (CIN) within this High-High group also had excellent survival. The High-High GS/CIN tumors were coupled with increased frequencies of Tbet+CD4+ T cells and central memory CD4+ T cells in the peripheral blood.
Conclusion
These novel findings identify the combination of CD8+ T cells and FOXP3+CD4+ T cells as a significant prognostic marker for GC patients, which also could potentially be targeted and applied in the combination therapy with immune checkpoint blockades in precision medicine.
中文翻译:

高维分析揭示了 T 细胞亚群在胃癌免疫微环境中的独特作用。
目的 为促进胃癌(GC)患者的疾病预后和提高精准免疫治疗,对肿瘤组织和外周血进行免疫细胞和分子分析相结合的综合研究。方法 使用多重免疫组织化学 (mIHC) 和转录组分析探索 GC 患者的结果与其肿瘤的免疫环境之间的关联。使用来自选定患者的补充外周血中的质谱流式细胞术 (CyTOF) 进一步检查了系统水平肿瘤中潜在的免疫功能障碍机制。具有 mIHC 和基因表达谱数据的 GC 队列也被用作验证队列。结果 GC 肿瘤中 CD4+FOXP3+ T 细胞密度增加与生存期延长相关。有趣的是,CD4+FOXP3+ T 细胞与 CD8+ T 细胞而非肿瘤细胞有密切的相互作用。高密度的 CD4+FOXP3+ T 细胞和 CD8+ T 细胞 (High-High) 独立预测延长的患者生存期。此外,干扰素-γ (IFN-γ) 基因特征和 PDL1 表达在该组中上调。重要的是,该高-高组中基因组稳定 (GS) 肿瘤和染色体不稳定 (CIN) 肿瘤的亚组也具有出色的存活率。高-高 GS/CIN 肿瘤与外周血中 Tbet+CD4+ T 细胞和中央记忆 CD4+ T 细胞的频率增加有关。结论 这些新发现将 CD8+ T 细胞和 FOXP3+CD4+ T 细胞的组合确定为 GC 患者的重要预后标志物,
更新日期:2020-05-05
中文翻译:

高维分析揭示了 T 细胞亚群在胃癌免疫微环境中的独特作用。
目的 为促进胃癌(GC)患者的疾病预后和提高精准免疫治疗,对肿瘤组织和外周血进行免疫细胞和分子分析相结合的综合研究。方法 使用多重免疫组织化学 (mIHC) 和转录组分析探索 GC 患者的结果与其肿瘤的免疫环境之间的关联。使用来自选定患者的补充外周血中的质谱流式细胞术 (CyTOF) 进一步检查了系统水平肿瘤中潜在的免疫功能障碍机制。具有 mIHC 和基因表达谱数据的 GC 队列也被用作验证队列。结果 GC 肿瘤中 CD4+FOXP3+ T 细胞密度增加与生存期延长相关。有趣的是,CD4+FOXP3+ T 细胞与 CD8+ T 细胞而非肿瘤细胞有密切的相互作用。高密度的 CD4+FOXP3+ T 细胞和 CD8+ T 细胞 (High-High) 独立预测延长的患者生存期。此外,干扰素-γ (IFN-γ) 基因特征和 PDL1 表达在该组中上调。重要的是,该高-高组中基因组稳定 (GS) 肿瘤和染色体不稳定 (CIN) 肿瘤的亚组也具有出色的存活率。高-高 GS/CIN 肿瘤与外周血中 Tbet+CD4+ T 细胞和中央记忆 CD4+ T 细胞的频率增加有关。结论 这些新发现将 CD8+ T 细胞和 FOXP3+CD4+ T 细胞的组合确定为 GC 患者的重要预后标志物,











































 京公网安备 11010802027423号
京公网安备 11010802027423号