当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Dynamic Interaction of n-Butane with Imidazolium-Based Ionic Liquids.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-19 , DOI: 10.1002/anie.202005991 Radha G Bhuin 1 , Leonhard Winter 1 , Matthias Lexow 1 , Florian Maier 1 , Hans-Peter Steinrück 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-19 , DOI: 10.1002/anie.202005991 Radha G Bhuin 1 , Leonhard Winter 1 , Matthias Lexow 1 , Florian Maier 1 , Hans-Peter Steinrück 1
Affiliation
|
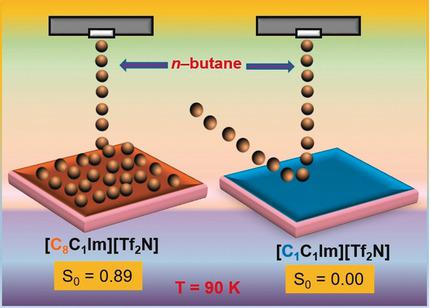
The impact of a reactant from the gas phase on the surface of a liquid and its transfer through this gas/liquid interface are crucial for various concepts applying ionic liquids (ILs) in catalysis. We investigated the first step of the adsorption dynamics of n‐butane on a series of 1‐alkyl‐3‐methylimidazolium bis(trifluoromethanesulfonyl)imide ILs ([Cn C1Im][Tf2N]; n =1, 2, 3, 8). Using a supersonic molecular beam in ultra‐high vacuum, the trapping of n‐butane on the frozen ILs was determined as a function of surface temperature, between 90 and 125 K. On the C8‐ and C3‐ILs, n‐butane adsorbs at 90 K with an initial trapping probability of ≈0.89. The adsorption energy increases with increasing length of the IL alkyl chain, whereas the ionic headgroups seem to interact only weakly with n‐butane. The absence of adsorption on the C1‐ and C2‐ILs is attributed to a too short residence time on the IL surface to form nuclei for condensation even at 90 K.
中文翻译:

正丁烷与咪唑基离子液体的动态相互作用
气相反应物对液体表面的影响及其通过该气/液界面的转移对于在催化中应用离子液体(IL)的各种概念至关重要。我们研究了正丁烷在一系列1-烷基-3-甲基咪唑鎓双(三氟甲磺酰基)酰亚胺ILs [[C n C 1 Im] [Tf 2 N]; n = 1,2, 3,8)。在超高真空下使用超声波分子束,确定了冷冻ILs上正丁烷的捕集量与表面温度的函数关系,介于90和125 K之间。在C 8-和C 3 -ILs上,n丁烷在90 K时吸附,初始俘获概率约为0.89。吸附能随着IL烷基链长度的增加而增加,而离子头基似乎只与正丁烷发生微弱的相互作用。C 1-和C 2 -IL上不存在吸附的原因是,即使在90 K时,在IL表面的停留时间也太短,无法形成可凝结的核。
更新日期:2020-05-19
中文翻译:

正丁烷与咪唑基离子液体的动态相互作用
气相反应物对液体表面的影响及其通过该气/液界面的转移对于在催化中应用离子液体(IL)的各种概念至关重要。我们研究了正丁烷在一系列1-烷基-3-甲基咪唑鎓双(三氟甲磺酰基)酰亚胺ILs [[C n C 1 Im] [Tf 2 N]; n = 1,2, 3,8)。在超高真空下使用超声波分子束,确定了冷冻ILs上正丁烷的捕集量与表面温度的函数关系,介于90和125 K之间。在C 8-和C 3 -ILs上,n丁烷在90 K时吸附,初始俘获概率约为0.89。吸附能随着IL烷基链长度的增加而增加,而离子头基似乎只与正丁烷发生微弱的相互作用。C 1-和C 2 -IL上不存在吸附的原因是,即使在90 K时,在IL表面的停留时间也太短,无法形成可凝结的核。











































 京公网安备 11010802027423号
京公网安备 11010802027423号