Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.jaci.2020.04.053 Su Han Lum 1 , Sabeena Selvarajah 2 , Angela Deya-Martinez 2 , Peter McNaughton 2 , Ali Sobh 2 , Sheila Waugh 3 , Shirelle Burton-Fanning 4 , Lisa Newton 3 , Julie Gandy 3 , Zohreh Nademi 5 , Stephen Owens 2 , Eleri Williams 2 , Marieke Emonts 5 , Terry Flood 2 , Andrew Cant 5 , Mario Abinun 6 , Sophie Hambleton 5 , Andrew R Gennery 5 , Mary Slatter 5
|
Background
Post hematopoietic cell transplantation (HCT) autoimmune cytopenia (AIC) is a potentially life-threatening complication, but studies focusing on large cohorts of patients transplanted for primary immunodeficiency are lacking.
Objectives
This study sought to determine the incidence, risk factors, and outcomes of post-HCT AIC and B-lymphocyte function following rituximab.
Methods
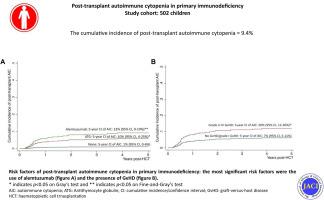
We retrospectively studied 502 children with primary immunodeficiency who were transplanted at our center between 1987 and 2018.
Results
Thirty-six patients (9%) developed post-HCT AIC, with a median onset of 6.5 months post-HCT. On univariate analysis, pre-HCT AIC, mismatched donor, alemtuzumab, anti-thymocyte antiglobulin, and acute and chronic graft versus host disease were significantly associated with post-HCT AIC. After multivariate analysis, alemtuzumab (subdistribution hazard ratio, 9.0; 95% CI, 1.50-54.0; P = .02) was independently associated with post-HCT AIC. Corticosteroid and high-dose intravenous immunoglobulin achieved remission in 50% (n = 18), additional rituximab led to remission in 25% (n = 9), and the remaining 25% were treated with a combination of various modalities including sirolimus (n = 5), bortezomib (n = 3), mycophenolate mofetil (n = 2), splenectomy (n = 2), and second HCT (n = 3). The mortality of post-HCT AIC reduced from 25% (4 of 16) prior to 2011 to 5% (1 of 20) after 2011. The median follow-up of 5.8 years (range, 0.4 to 29.1 years) showed that 26 of 30 survivors (87%) were in complete remission, and 4 were in remission with ongoing sirolimus and low-dose steroids. Of the 17 who received rituximab, 7 had B-lymphocyte recovery, 5 had persistent low B-lymphocyte count and remained on intravenous immunoglobulin replacement, 2 had second HCT, and 3 died.
Conclusions
The frequency of post HCT AIC in our cohort was 9%, and the most significant risk factors for its occurrence were the presence of graft versus host disease and the use of alemtuzumab.
中文翻译:

造血细胞移植后原发性免疫缺陷患者自身免疫性血细胞减少的结果。
背景
造血后细胞移植(HCT)自身免疫性血细胞减少症(AIC)是可能危及生命的并发症,但缺乏针对大量因原发性免疫缺陷而移植的患者的研究。
目标
这项研究试图确定利妥昔单抗后HCT后AIC和B淋巴细胞功能的发生率,危险因素和结果。
方法
我们回顾性研究了1987年至2018年之间在我们中心移植的502例原发性免疫缺陷儿童。
结果
三十六名患者(9%)发生于HCT后AIC,中位发病时间为HCT后6.5个月。单因素分析显示,HCT前AIC,不匹配的供体,阿仑单抗,抗胸腺细胞抗球蛋白以及急性和慢性移植物抗宿主病与HCT后AIC显着相关。经过多变量分析后,阿来珠单抗(亚分布危险比为9.0; 95%CI为1.50-54.0;P = .02)与HCT后AIC独立相关。皮质类固醇和大剂量静脉免疫球蛋白的缓解率达50%(n = 18),另外的利妥昔单抗导致的缓解率达25%(n = 9),其余25%的患者接受了包括西罗莫司(n = 5),硼替佐米(n = 3),霉酚酸酯(n = 2),脾切除术(n = 2)和第二次HCT(n = 3)。HCT后AIC的死亡率从2011年之前的25%(16个中的4个)降低到2011年之后的5%(20个中的1个)。中位随访5.8年(范围0.4到29.1年)显示,有26个30名幸存者(87%)完全缓解,4名持续西罗莫司和低剂量类固醇缓解。在接受利妥昔单抗的17例患者中,有7例具有B淋巴细胞恢复功能,有5例具有持续的低B淋巴细胞计数,并且仍在静脉内补充免疫球蛋白,
结论
HCT后AIC在我们队列中的发生率为9%,其发生的最重要危险因素是移植物抗宿主病的存在以及使用alemtuzumab。











































 京公网安备 11010802027423号
京公网安备 11010802027423号