Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.freeradbiomed.2020.05.007 Luke Carroll 1 , Kelly Gardiner 2 , Marta Ignasiak 3 , Jeppe Holmehave 4 , Shingo Shimodaira 5 , Thomas Breitenbach 4 , Michio Iwaoka 6 , Peter R Ogilby 4 , David I Pattison 7 , Michael J Davies 2
|
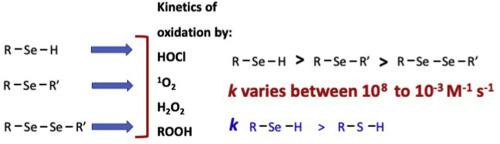
Selenium compounds have been identified as potential oxidant scavengers for biological applications due to the nucleophilicity of Se, and the ease of oxidation of the selenium centre. Previous studies have reported apparent second order rate constants for a number of oxidants (e.g. HOCl, ONOOH) with some selenium species, but these data are limited. Here we provide apparent second order rate constants for reaction of selenols (RSeH), selenides (RSeR′) and diselenides (RSeSeR’) with biologically-relevant oxidants (HOCl, H2O2, other peroxides) as well as overall consumption data for the excited state species singlet oxygen (1O2). Selenols show very high reactivity with HOCl and 1O2, with rate constants > 108 M−1 s−1, whilst selenides and diselenides typically react with rate constants one- (selenides) or two- (diselenides) orders of magnitude slower. Rate constants for reaction of diselenides with H2O2 and other hydroperoxides are much slower, with k for H2O2 being <1 M−1 s−1, and for amino acid and peptide hydroperoxides ~102 M−1 s−1. The rate constants determined for HOCl and 1O2 with these selenium species are greater than, or similar to, rate constants for amino acid side chains on proteins, including the corresponding sulfur-centered species (Cys and Met), suggesting that selenium containing compounds may be effective oxidant scavengers. Some of these reactions may be catalytic in nature due to ready recycling of the oxidized selenium species. These data may aid the development of highly efficacious, and catalytic, oxidant scavengers.
中文翻译:

含硒化合物与氧化剂的相互作用动力学。
由于硒的亲核性和硒中心的易氧化性,硒化合物已被鉴定为生物应用中潜在的氧化剂清除剂。先前的研究已经报道了许多氧化剂(例如HOCl,ONOOH)和某些硒物种的明显的二阶速率常数,但是这些数据是有限的。在这里,我们提供了硒醇(RSeH),硒化物(RSeR')和二硒化物(RSeSeR')与生物相关氧化剂(HOCl,H 2 O 2,其他过氧化物)的反应的明显的二阶速率常数,以及激发态物种单线态氧(1 O 2)。硒醇与HOCl和1 O 2的反应性很高,其速率常数> 10 8 M -1 s -1,而硒化物和二硒化物的反应速率常数通常慢一个(硒化物)或两个(二硒化物)量级。为用H diselenides的反应速率常数2 ö 2和其它氢过氧化物慢得多,其中k用于h 2 ö 2是<1M的-1 小号-1,以及用于氨基酸和肽过氧化物〜10个2 中号-1 小号- 1。确定HOCl和1 O 2的速率常数含硒化合物的含硒量大于或等于蛋白质上氨基酸侧链的速率常数,包括相应的以硫为中心的种类(Cys和Met),表明含硒化合物可能是有效的氧化剂清除剂。这些反应中的某些反应由于氧化硒物质的现成再循环而本质上可能是催化的。这些数据可能有助于开发高效,催化性的氧化剂清除剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号