当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of C-mannosylation in the secretion of mindin.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.bbagen.2020.129632 Yoko Inai 1 , Kana Ueda 1 , In-Sook Lee Matsui 1 , Michiko Tajiri 2 , Shiho Minakata 1 , Yoshinao Wada 2 , Yoshito Ihara 1
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.bbagen.2020.129632 Yoko Inai 1 , Kana Ueda 1 , In-Sook Lee Matsui 1 , Michiko Tajiri 2 , Shiho Minakata 1 , Yoshinao Wada 2 , Yoshito Ihara 1
Affiliation
|
BACKGROUND
Mindin (spondin2), a secretory protein related to neural development and immunity, is a member of thrombospondin type I repeat (TSR) superfamily proteins, and has a unique glycosylation of C-mannosylation in its structure. However, it remains unclear whether C-mannosylation plays a functional role in the biosynthesis of mindin in cells.
METHODS
Protein C-mannosylation was analyzed by mass spectrometry. Mindin expression was examined by immunoblot and immunofluorescence analyses in COS-7 cells transfected with the expression vectors for wild type (mindin-WT) or C-mannosylation-defective mutant of mindin (mindin-mutF). The redox status was examined in mindin by using 4-acetoamide-4'-maleimidylstilbene-2,2'-disulfonate.
RESULTS
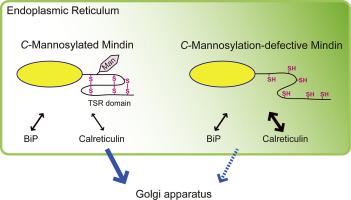
When mindin cDNA was expressed in COS-7 cells, C-mannosylation of mindin was confirmed at Trp257 by mass spectrometry. In cells expressing a mindin-mutF, secretion of the mutant was significantly inhibited compared with mindin-WT. In immunofluorescence analysis, mindin-mutF was accumulated in the endoplasmic reticulum (ER), whereas mindin-WT was detected in the Golgi. In addition, mindin-mutF showed an enhanced interaction with calreticulin, an ER-resident chaperone, in cells. In cells, reduced forms were increased in mindin-mutF, compared with a mostly oxidized form of mindin-WT. In the presence of chemical chaperones such as dimethylsulfoxide or 4-phenylbutyrate, inhibited secretion of mindin-mutF was ameliorated in cells, although redox-dependent folding was not affected.
CONCLUSIONS
C-Mannosylation of mindin facilitates its secretion especially through modulating disulfide bond formation in mindin in cells.
GENERAL SIGNIFICANCE
These results suggest that C-mannosylation plays a functional role in the redox-dependent folding and transport of TSR superfamily proteins in cells.
中文翻译:

C-甘露糖基化在薄荷素分泌中的作用。
背景Mindin(spondin2)是与神经发育和免疫相关的分泌蛋白,是血小板反应蛋白I型重复(TSR)超家族蛋白的成员,并且在其结构中具有独特的C-甘露糖基糖基化。但是,尚不清楚C-甘露糖基化是否在细胞中脑神经素的生物合成中发挥功能性作用。方法通过质谱分析蛋白质C-甘露糖基化。通过用野生型(mindin-WT)或Cindin的C-甘露糖基化缺陷型突变体(mindin-mutF)的表达载体转染的COS-7细胞,通过免疫印迹和免疫荧光分析检查mindin的表达。通过使用4-乙酰胺-4'-马来酰亚胺基苯乙烯-2,2'-二磺酸盐在脑中检查氧化还原状态。结果当mindin cDNA在COS-7细胞中表达时,在Trp257上通过质谱法证实了脑蛋白的C-甘露糖基化。在表达minin-mutF的细胞中,与minin-WT相比,突变体的分泌受到显着抑制。在免疫荧光分析中,mindin-mutF积累在内质网(ER)中,而mindin-WT在高尔基体中检测到。此外,mindin-mutF在细胞中与钙网蛋白(一种内质网内的伴侣分子)的相互作用增强。在细胞中,与大部分氧化形式的minin-WT相比,minin-mutF的还原形式增加了。在化学分子伴侣如二甲基亚砜或4-苯基丁酸酯的存在下,虽然未影响氧化还原依赖性折叠,但改善了细胞中mindin-mutF的抑制分泌。结论薄荷脑的C-甘露糖基化促进了其分泌,特别是通过调节细胞中的薄荷脑中的二硫键形成。一般意义这些结果表明,C-甘露糖基化在细胞中TSR超家族蛋白的氧化还原依赖性折叠和运输中起功能性作用。
更新日期:2020-05-19
中文翻译:

C-甘露糖基化在薄荷素分泌中的作用。
背景Mindin(spondin2)是与神经发育和免疫相关的分泌蛋白,是血小板反应蛋白I型重复(TSR)超家族蛋白的成员,并且在其结构中具有独特的C-甘露糖基糖基化。但是,尚不清楚C-甘露糖基化是否在细胞中脑神经素的生物合成中发挥功能性作用。方法通过质谱分析蛋白质C-甘露糖基化。通过用野生型(mindin-WT)或Cindin的C-甘露糖基化缺陷型突变体(mindin-mutF)的表达载体转染的COS-7细胞,通过免疫印迹和免疫荧光分析检查mindin的表达。通过使用4-乙酰胺-4'-马来酰亚胺基苯乙烯-2,2'-二磺酸盐在脑中检查氧化还原状态。结果当mindin cDNA在COS-7细胞中表达时,在Trp257上通过质谱法证实了脑蛋白的C-甘露糖基化。在表达minin-mutF的细胞中,与minin-WT相比,突变体的分泌受到显着抑制。在免疫荧光分析中,mindin-mutF积累在内质网(ER)中,而mindin-WT在高尔基体中检测到。此外,mindin-mutF在细胞中与钙网蛋白(一种内质网内的伴侣分子)的相互作用增强。在细胞中,与大部分氧化形式的minin-WT相比,minin-mutF的还原形式增加了。在化学分子伴侣如二甲基亚砜或4-苯基丁酸酯的存在下,虽然未影响氧化还原依赖性折叠,但改善了细胞中mindin-mutF的抑制分泌。结论薄荷脑的C-甘露糖基化促进了其分泌,特别是通过调节细胞中的薄荷脑中的二硫键形成。一般意义这些结果表明,C-甘露糖基化在细胞中TSR超家族蛋白的氧化还原依赖性折叠和运输中起功能性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号