Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-05-19 , DOI: 10.1016/j.actbio.2020.05.014 Sebastian Kollenda 1 , Mathis Kopp 1 , Jasmin Wens 1 , Johannes Koch 2 , Nina Schulze 2 , Chrisovalantis Papadopoulos 3 , Robert Pöhler 3 , Hemmo Meyer 3 , Matthias Epple 1
|
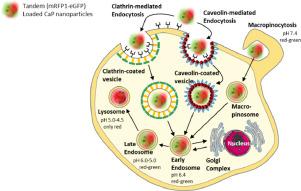
Calcium phosphate nanoparticles (100 nm) were fluorescently labelled with poly(ethyleneimine) (PEIATTO490LS; red fluorescence). They were loaded with a Tandem fusion protein consisting of mRFP1-eGFP (red and green fluorescence in the same molecule)that acts as smart biological pH sensor to trace nanoparticles inside cells. Its fluorescence is also coupled to the structural integrity of the protein, i.e. it is also a label for a successful delivery of a functional protein into the cell. At pH 7.4, the fluorescence of both proteins (red and green) is detectable. At a pH of 4.5-5 inside the lysosomes, the green fluorescence is quenched due to the protonation of the eGFP chromophore, but the pH-independent red fluorescence of mRFP1 remains. The nanoparticles were taken up by cells (cell lines: HeLa, Caco-2 and A549) via endocytic pathways and then directed to lysosomes. Time-resolved confocal laser scanning microscopy confirmed mRFP1 and nanoparticles co-localizing with lysosomes. The fluorescence of eGFP was only detectable outside lysosomes, i.e. most likely inside early endosomes or at the cell membrane during the uptake, indicating the neutral pH at these locations. The Tandem fusion protein provides a versatile platform to follow the intracellular pathway of bioactive nanocarriers, e.g. therapeutic proteins. The transfection with a Tandem-encoding plasmid by calcium phosphate nanoparticles led to an even intracellular protein distribution in cytosol and nucleoplasm, i.e. very different from direct protein uptake. Neither dissolved protein nor dissolved plasmid DNA were taken up by the cells, underscoring the necessity for a suitable carrier like a nanoparticle.
Statement of Significance
A pH-sensitive protein ("tandem") was used to follow the pathway of calcium phosphate nanoparticles. This protein consists of a pH-sensitive fluorophore (eGFP; green) and a pH-independent fluorophore (mRFP1; red). This permits to follow the pathway of a nanoparticle inside a cell. At a low pH inside an endolysosome, the green fluorescence vanishes but the red fluorescence persists. This is also a very useful model for the delivery of therapeutic proteins into cells. The delivery by nanoparticles was compared with the protein expression after cell transfection with plasmid DNA encoding for the tandem protein. High-resolution image analysis gave quantitative data on the intracellular protein distribution.
中文翻译:

一种pH敏感的荧光蛋白传感器,可追踪磷酸钙纳米颗粒进入细胞的途径。
用聚乙烯亚胺(PEI ATTO490LS)对磷酸钙纳米颗粒(100 nm)进行荧光标记; 红色荧光)。它们装有由mRFP1-eGFP(同一分子中的红色和绿色荧光)组成的串联融合蛋白,可作为智能生物pH传感器来追踪细胞内的纳米颗粒。它的荧光也与蛋白质的结构完整性有关,即它也是功能性蛋白质成功递送到细胞中的标记。在pH 7.4时,两种蛋白质(红色和绿色)的荧光都可以检测到。在溶酶体内部的pH值为4.5-5时,由于eGFP发色团的质子化,绿色荧光被猝灭,但是mRFP1的pH无关红色荧光仍然存在。纳米颗粒通过细胞内吞途径被细胞(细胞系:HeLa,Caco-2和A549)吸收,然后被导向溶酶体。时间分辨共聚焦激光扫描显微镜证实了mRFP1和纳米粒子与溶酶体共定位。eGFP的荧光仅在溶酶体外部可检测到,即最有可能在早期内涵体内部或摄取期间在细胞膜处检测到,表明这些位置的pH为中性。串联融合蛋白提供了遵循生物活性纳米载体,例如治疗性蛋白的细胞内途径的通用平台。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。eGFP的荧光只能在溶酶体外部检测到,即最有可能在早期内涵体内部或摄取期间在细胞膜处检测到,表明这些位置的pH为中性。串联融合蛋白提供了遵循生物活性纳米载体,例如治疗性蛋白的细胞内途径的通用平台。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。eGFP的荧光只能在溶酶体外部检测到,即最有可能在早期内涵体内部或摄取期间在细胞膜处检测到,表明这些位置的pH为中性。串联融合蛋白提供了遵循生物活性纳米载体,例如治疗性蛋白的细胞内途径的通用平台。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。指示这些位置的中性pH。串联融合蛋白提供了遵循生物活性纳米载体,例如治疗性蛋白的细胞内途径的通用平台。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。指示这些位置的中性pH。串联融合蛋白提供了遵循生物活性纳米载体,例如治疗性蛋白的细胞内途径的通用平台。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。磷酸钙纳米颗粒用编码串联编码的质粒转染导致细胞内蛋白质在胞浆和核质中的分布均匀,即与直接蛋白质摄取截然不同。细胞既不吸收溶解的蛋白质,也不吸收溶解的质粒DNA,这强调了需要合适的载体(如纳米颗粒)的必要性。
重要声明
pH敏感蛋白(“串联”)用于遵循磷酸钙纳米颗粒的路径。该蛋白由pH敏感的荧光团(eGFP;绿色)和pH无关的荧光团(mRFP1;红色)组成。这允许遵循细胞内纳米颗粒的路径。在溶酶体内部的低pH值下,绿色荧光消失,但红色荧光持续存在。这也是用于将治疗性蛋白质递送到细胞中的非常有用的模型。将纳米颗粒的递送与用编码串联蛋白的质粒DNA转染细胞后的蛋白表达进行比较。高分辨率图像分析给出了细胞内蛋白质分布的定量数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号