当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A fundamental study of the thermoelectrochemistry of ferricyanide/ferrocyanide: cation, concentration, ratio, and heterogeneous and homogeneous electrocatalysis effects in thermogalvanic cells
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-05-18 , DOI: 10.1039/d0se00440e Mark A. Buckingham 1, 2, 3, 4, 5 , Samer Hammoud 1, 2, 3, 4, 5 , Huanxin Li 1, 2, 3, 4, 5 , Conor J. Beale 1, 2, 3, 4, 5 , Jason T. Sengel 1, 2, 3, 4, 5 , Leigh Aldous 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-05-18 , DOI: 10.1039/d0se00440e Mark A. Buckingham 1, 2, 3, 4, 5 , Samer Hammoud 1, 2, 3, 4, 5 , Huanxin Li 1, 2, 3, 4, 5 , Conor J. Beale 1, 2, 3, 4, 5 , Jason T. Sengel 1, 2, 3, 4, 5 , Leigh Aldous 1, 2, 3, 4, 5
Affiliation
|
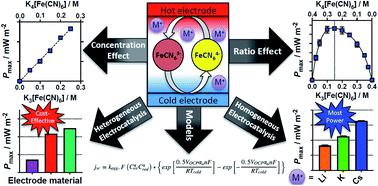
Thermogalvanic cells typically utilise equimolar concentrations of the oxidised and reduced states of a redox couple in solution, sandwiched between two electrodes at dissimilar temperatures; entropy drives redox processes to occur at these electrodes, generating a potential difference and a current. However, significant gaps still exist in fundamental data and understanding of these ‘thermocells’. In this study, thermocells based upon potassium ferricyanide, K3[Fe(CN)6], and potassium ferrocyanide, K4[Fe(CN)6], were investigated. The ratio of the oxidised and reduced states were systematically varied, and this had a significant effect upon the power produced; notably maximum power did not correspond to the equimolar ratio. A concentration study using equimolar ratios was also performed. Trends in the potential generated as a function of temperature (or ‘Seebeck coefficient’) were rationalised by the Nernst equation and Debye–Hückel theory. The trends in the current and the electrical power produced were successfully modelled using the Butler–Volmer equation. The effects of heterogeneous electrocatalysis were also explored (using platinum and two types of graphite) as well as homogeneous electrocatalysis, by the direct addition of alkali metal salts (as lithium, sodium, potassium, rubidium and caesium chlorides and sulphates). Clear trends were observed, and homogeneous and heterogeneous electrocatalysis had an additive effect when combined. Addition of CsCl was able to boost the maximum power output by upto ca. 80%, via both an increased Seebeck coefficient (through altered solvation) and through increased current (via homogeneous electrocatalysis of electron transfer). Finally, a limited economic comparison was performed, which highlights how the use of non-stoichiometric ratios of the redox couple could improve the cost-per-power value of the systems.
中文翻译:

铁氰化物/亚铁氰化物的热电化学的基础研究:阳离子,浓度,比例以及热原电池中的非均相和均相电催化作用
热原电池通常利用等摩尔浓度的溶液中氧化还原对的氧化态和还原态,在不同温度下夹在两个电极之间。熵驱动氧化还原过程发生在这些电极上,从而产生电势差和电流。但是,在基础数据和对这些“热电池”的理解上仍然存在巨大差距。在这项研究中,基于铁氰化钾K 3 [Fe(CN)6 ]和亚铁氰化钾K 4 [Fe(CN)6的热电池],进行了调查。系统改变了氧化态和还原态的比率,这对产生的功率有重大影响;明显地,最大功率不等于等摩尔比。还进行了使用等摩尔比的浓度研究。通过Nernst方程和Debye-Hückel理论合理化了随温度(或“塞贝克系数”)而变化的电势趋势。使用Butler-Volmer方程成功地模拟了电流和产生的电力的趋势。通过直接添加碱金属盐(如锂,钠,钾、,和铯的氯化物和硫酸盐),还探索了非均相电催化作用(使用铂和两种类型的石墨)以及均相电催化作用。观察到明显的趋势,并且均相和非均相电催化结合时具有加和效应。添加CsCl可以将最大功率输出提高到ca. 80%,通过两者的增加的塞贝克系数(通过改变溶剂化),并通过增加的电流(经由电子传递的均匀电催化)。最后,进行了有限的经济比较,突出了氧化还原对的非化学计量比的使用如何提高系统的每功率成本价值。
更新日期:2020-06-30
中文翻译:

铁氰化物/亚铁氰化物的热电化学的基础研究:阳离子,浓度,比例以及热原电池中的非均相和均相电催化作用
热原电池通常利用等摩尔浓度的溶液中氧化还原对的氧化态和还原态,在不同温度下夹在两个电极之间。熵驱动氧化还原过程发生在这些电极上,从而产生电势差和电流。但是,在基础数据和对这些“热电池”的理解上仍然存在巨大差距。在这项研究中,基于铁氰化钾K 3 [Fe(CN)6 ]和亚铁氰化钾K 4 [Fe(CN)6的热电池],进行了调查。系统改变了氧化态和还原态的比率,这对产生的功率有重大影响;明显地,最大功率不等于等摩尔比。还进行了使用等摩尔比的浓度研究。通过Nernst方程和Debye-Hückel理论合理化了随温度(或“塞贝克系数”)而变化的电势趋势。使用Butler-Volmer方程成功地模拟了电流和产生的电力的趋势。通过直接添加碱金属盐(如锂,钠,钾、,和铯的氯化物和硫酸盐),还探索了非均相电催化作用(使用铂和两种类型的石墨)以及均相电催化作用。观察到明显的趋势,并且均相和非均相电催化结合时具有加和效应。添加CsCl可以将最大功率输出提高到ca. 80%,通过两者的增加的塞贝克系数(通过改变溶剂化),并通过增加的电流(经由电子传递的均匀电催化)。最后,进行了有限的经济比较,突出了氧化还原对的非化学计量比的使用如何提高系统的每功率成本价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号