当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Environmentally Friendly and Regioselective One-Pot Synthesis of Imines and Oxazolidines Serinol Derivatives and Their Use for Rubber Cross-Linking
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-18 , DOI: 10.1021/acssuschemeng.0c01603 Vincenzina Barbera 1 , Gabriella Leonardi 1 , Antonio Marco Valerio 1 , Lucia Rubino 1 , Shuquan Sun 2 , Antonino Famulari 1 , Maurizio Galimberti 1 , Attilio Citterio 1 , Roberto Sebastiano 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-18 , DOI: 10.1021/acssuschemeng.0c01603 Vincenzina Barbera 1 , Gabriella Leonardi 1 , Antonio Marco Valerio 1 , Lucia Rubino 1 , Shuquan Sun 2 , Antonino Famulari 1 , Maurizio Galimberti 1 , Attilio Citterio 1 , Roberto Sebastiano 1
Affiliation
|
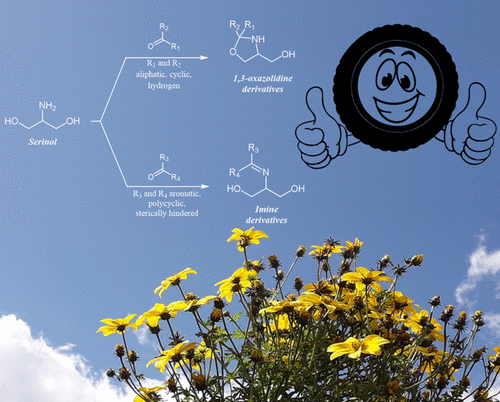
High-yield regioselective synthesis of imines and oxazolidines derivatives of 2-amino-1,3-propandiol (serinol) was achieved by performing the reaction with aldehydes and ketones, in the absence of solvents and catalysts. Only imines were obtained when the carbonyl compound was aromatic and/or sterically hindered and when conjugated double bonds were formed. 1,3-Oxazolidines were specifically obtained with either aldehydes or ketones with limited steric hindrance. The “green” reaction conditions here adopted for the synthesis of these classes of derivatives are not due to the structural and functional peculiarity of the serinol as a reactant and can also be extended to lipophilic amines with the same good results in terms of yield and selectivity. A revision of the mechanism typically accepted in the presence of solvent and catalysis is proposed, and the quantum mechanics calculations applied to some derivatives are in good agreement with the proposed rationalizations of the selectivity observed. Serinol itself and the imine and oxazolidine derivatives were used, in place of guanidine, as accelerators in compounds based on diene rubbers and silica, suitable for application in tire treads with low environmental impact. Efficient sulfur-based cross-linking and composites with a low dissipation of energy were obtained. The oxazolidine and imine appear to act as protective groups of the serinol primary amine. This work paves the way for the selective synthesis of biosourced families of chemicals, which could be used for large-scale applications, such as the one in rubber compounds, replacing toxic oil-based chemicals.
中文翻译:

亚胺和恶唑烷丝氨醇衍生物的环境友好和区域选择性一锅合成及其在橡胶交联中的应用
在没有溶剂和催化剂的情况下,通过与醛和酮进行反应,可以实现2-氨基-1,3-丙二醇(丝氨醇)的亚胺和恶唑烷衍生物的高产率区域选择性合成。当羰基化合物是芳族的和/或位阻的并且形成共轭双键时,仅获得亚胺。1,3-恶唑烷是通过具有有限空间位阻的醛或酮专门获得的。此处用于合成这类衍生物的“绿色”反应条件不是由于丝氨醇作为反应物的结构和功能的特殊性,还可以扩展到亲脂性胺,在产率和选择性方面都具有相同的良好结果。提出了对通常在溶剂和催化条件下可接受的机理的修订,应用于某些导数的量子力学计算与所观察到的选择性的合理化理论相吻合。丝氨醇本身以及亚胺和恶唑烷衍生物代替胍用作基于二烯橡胶和二氧化硅的化合物中的促进剂,适用于对环境影响小的轮胎胎面。获得了一种低能耗的高效硫基交联和复合材料。恶唑烷和亚胺似乎是丝氨醇伯胺的保护基。这项工作为选择性合成生物来源的化学品家族铺平了道路,这些化学品可用于大规模应用,例如橡胶化合物中的一种,代替有毒的油基化学品。
更新日期:2020-06-29
中文翻译:

亚胺和恶唑烷丝氨醇衍生物的环境友好和区域选择性一锅合成及其在橡胶交联中的应用
在没有溶剂和催化剂的情况下,通过与醛和酮进行反应,可以实现2-氨基-1,3-丙二醇(丝氨醇)的亚胺和恶唑烷衍生物的高产率区域选择性合成。当羰基化合物是芳族的和/或位阻的并且形成共轭双键时,仅获得亚胺。1,3-恶唑烷是通过具有有限空间位阻的醛或酮专门获得的。此处用于合成这类衍生物的“绿色”反应条件不是由于丝氨醇作为反应物的结构和功能的特殊性,还可以扩展到亲脂性胺,在产率和选择性方面都具有相同的良好结果。提出了对通常在溶剂和催化条件下可接受的机理的修订,应用于某些导数的量子力学计算与所观察到的选择性的合理化理论相吻合。丝氨醇本身以及亚胺和恶唑烷衍生物代替胍用作基于二烯橡胶和二氧化硅的化合物中的促进剂,适用于对环境影响小的轮胎胎面。获得了一种低能耗的高效硫基交联和复合材料。恶唑烷和亚胺似乎是丝氨醇伯胺的保护基。这项工作为选择性合成生物来源的化学品家族铺平了道路,这些化学品可用于大规模应用,例如橡胶化合物中的一种,代替有毒的油基化学品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号