当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoelectrochemical Nitrogen Reduction to Ammonia on Cupric and Cuprous Oxide Photocathodes
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-05-18 , DOI: 10.1021/acsenergylett.0c00711 Youn Jeong Jang 1, 2 , Ann E. Lindberg 1 , Margaret A. Lumley 1 , Kyoung-Shin Choi 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-05-18 , DOI: 10.1021/acsenergylett.0c00711 Youn Jeong Jang 1, 2 , Ann E. Lindberg 1 , Margaret A. Lumley 1 , Kyoung-Shin Choi 1
Affiliation
|
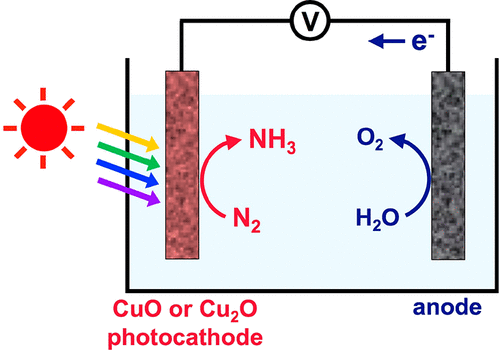
Photoelectrochemical N2 reduction enables the production of NH3 under ambient conditions using water as the hydrogen source. Furthermore, by utilizing solar energy, photoelectrochemical N2 reduction can significantly reduce the amount of energy required for N2 reduction. In this study, photoelectrochemical N2 reduction was investigated using CuO and Cu2O photocathodes that are known to be poorly catalytic for water reduction, the major reaction competing with N2 reduction. When tested under simulated solar illumination with isotopically labeled 15N2 in a 0.1 M KOH solution, the CuO and Cu2O photocathodes produced 15NH3 with Faradaic efficiencies of 17% and 20% at 0.6 and 0.4 V vs the reversible hydrogen electrode, respectively. These potentials are significantly more positive than the thermodynamic reduction potential of N2, which demonstrates how the use of photoexcited electrons in the CuO and Cu2O photocathodes can reduce the amount of energy required for NH3 production. The use of photoexcited electrons in these photocathodes for N2 reduction, water reduction, and photocorrosion was carefully examined.
中文翻译:

在氧化铜和氧化亚铜光电阴极上将光电化学氮还原为氨
光电化学N 2的还原能够在环境条件下使用水作为氢源生产NH 3。此外,通过利用太阳能,光电化学Ñ 2减少可以减少显著能量的N个所需要的量2减少。在这项研究中,使用已知对水还原反应催化不良的CuO和Cu 2 O光电阴极研究了光电化学法对N 2的还原,主要反应与N 2还原竞争。当在0.1 M KOH溶液中用同位素标记的15 N 2在模拟太阳光照下进行测试时,CuO和Cu 2与可逆氢电极相比,O光电阴极在0.6和0.4 V时分别产生15 NH 3的法拉第效率,分别为17%和20%。这些电势比N 2的热力学还原电势明显更正,这表明在CuO和Cu 2 O光电阴极中使用光激发电子如何减少NH 3产生所需的能量。仔细检查了在这些光电阴极中使用光激发电子来还原N 2,水还原和光腐蚀。
更新日期:2020-05-18
中文翻译:

在氧化铜和氧化亚铜光电阴极上将光电化学氮还原为氨
光电化学N 2的还原能够在环境条件下使用水作为氢源生产NH 3。此外,通过利用太阳能,光电化学Ñ 2减少可以减少显著能量的N个所需要的量2减少。在这项研究中,使用已知对水还原反应催化不良的CuO和Cu 2 O光电阴极研究了光电化学法对N 2的还原,主要反应与N 2还原竞争。当在0.1 M KOH溶液中用同位素标记的15 N 2在模拟太阳光照下进行测试时,CuO和Cu 2与可逆氢电极相比,O光电阴极在0.6和0.4 V时分别产生15 NH 3的法拉第效率,分别为17%和20%。这些电势比N 2的热力学还原电势明显更正,这表明在CuO和Cu 2 O光电阴极中使用光激发电子如何减少NH 3产生所需的能量。仔细检查了在这些光电阴极中使用光激发电子来还原N 2,水还原和光腐蚀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号