当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Guided Optimization of Inhibitors of Acetyltransferase Eis from Mycobacterium tuberculosis.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-05-18 , DOI: 10.1021/acschembio.0c00184 Ankita Punetha 1 , Huy X Ngo 1 , Selina Y L Holbrook 1 , Keith D Green 1 , Melisa J Willby 2 , Shilah A Bonnett 3 , Kyle Krieger 3, 4 , Emily K Dennis 1 , James E Posey 2 , Tanya Parish 3, 4 , Oleg V Tsodikov 1 , Sylvie Garneau-Tsodikova 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-05-18 , DOI: 10.1021/acschembio.0c00184 Ankita Punetha 1 , Huy X Ngo 1 , Selina Y L Holbrook 1 , Keith D Green 1 , Melisa J Willby 2 , Shilah A Bonnett 3 , Kyle Krieger 3, 4 , Emily K Dennis 1 , James E Posey 2 , Tanya Parish 3, 4 , Oleg V Tsodikov 1 , Sylvie Garneau-Tsodikova 1
Affiliation
|
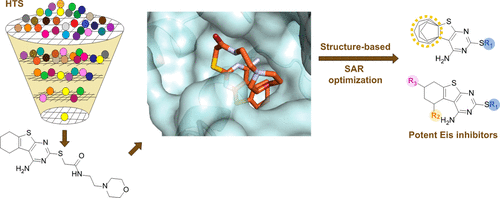
The enhanced intracellular survival (Eis) protein of Mycobacterium tuberculosis (Mtb) is a versatile acetyltransferase that multiacetylates aminoglycoside antibiotics abolishing their binding to the bacterial ribosome. When overexpressed as a result of promoter mutations, Eis causes drug resistance. In an attempt to overcome the Eis-mediated kanamycin resistance of Mtb, we designed and optimized structurally unique thieno[2,3-d]pyrimidine Eis inhibitors toward effective kanamycin adjuvant combination therapy. We obtained 12 crystal structures of enzyme–inhibitor complexes, which guided our rational structure-based design of 72 thieno[2,3-d]pyrimidine analogues divided into three families. We evaluated the potency of these inhibitors in vitro as well as their ability to restore the activity of kanamycin in a resistant strain of Mtb, in which Eis was upregulated. Furthermore, we evaluated the metabolic stability of 11 compounds in vitro. This study showcases how structural information can guide Eis inhibitor design.
中文翻译:

结核分枝杆菌乙酰转移酶Eis抑制剂的结构指导优化。
结核分枝杆菌(Mtb)的增强的细胞内存活(Eis)蛋白是一种多功能的乙酰转移酶,可对氨基糖苷类抗生素进行多乙酰化,从而消除了与细菌核糖体的结合。当由于启动子突变而过表达时,Eis引起耐药性。为了克服Eis介导的Mtb的卡那霉素耐药性,我们设计并优化了结构独特的硫代[2,3- d ]嘧啶Eis抑制剂,以实现有效的卡那霉素佐剂联合治疗。我们获得了12种酶抑制剂复合物的晶体结构,这指导了我们合理的基于72 thieno [2,3- d]嘧啶类似物分为三个家族。我们评估了这些抑制剂在体外的效力,以及它们在抗性Mtb菌株中恢复卡那霉素活性的能力,其中Eis被上调。此外,我们评估了11种化合物的体外代谢稳定性。这项研究展示了结构信息如何指导Eis抑制剂的设计。
更新日期:2020-06-19
中文翻译:

结核分枝杆菌乙酰转移酶Eis抑制剂的结构指导优化。
结核分枝杆菌(Mtb)的增强的细胞内存活(Eis)蛋白是一种多功能的乙酰转移酶,可对氨基糖苷类抗生素进行多乙酰化,从而消除了与细菌核糖体的结合。当由于启动子突变而过表达时,Eis引起耐药性。为了克服Eis介导的Mtb的卡那霉素耐药性,我们设计并优化了结构独特的硫代[2,3- d ]嘧啶Eis抑制剂,以实现有效的卡那霉素佐剂联合治疗。我们获得了12种酶抑制剂复合物的晶体结构,这指导了我们合理的基于72 thieno [2,3- d]嘧啶类似物分为三个家族。我们评估了这些抑制剂在体外的效力,以及它们在抗性Mtb菌株中恢复卡那霉素活性的能力,其中Eis被上调。此外,我们评估了11种化合物的体外代谢稳定性。这项研究展示了结构信息如何指导Eis抑制剂的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号