当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and crystallographic, spectroscopic and computational characterization of 3,3',4,4'-substituted biphenyls: effects of OR substituents on the intra-ring torsion angle.
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2020-05-18 , DOI: 10.1107/s2052520620004102 Nahir Vadra 1 , Sebastian A Suarez 1 , Leonardo D Slep 1 , Veronica E Manzano 1 , Emilia B Halac 2 , Ricardo F Baggio 2 , Fabio D Cukiernik 1
Acta Crystallographica Section B ( IF 1.3 ) Pub Date : 2020-05-18 , DOI: 10.1107/s2052520620004102 Nahir Vadra 1 , Sebastian A Suarez 1 , Leonardo D Slep 1 , Veronica E Manzano 1 , Emilia B Halac 2 , Ricardo F Baggio 2 , Fabio D Cukiernik 1
Affiliation
|
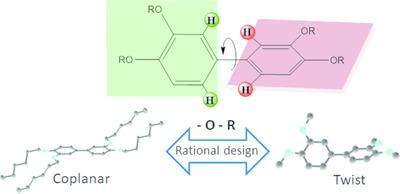
Presented here are the synthesis, characterization and study (using single crystal X‐ray diffraction, Raman scattering, quantum mechanics calculations) of the structures of a series of biphenyls substituted in positions 3, 3′, 4 and 4′ with a variety of R (R = methyl, acetyl, hexyl) groups connected to the biphenyl core through oxygen atoms. The molecular conformation, particularly the torsion angle between aromatic rings has been extensively studied both in the solid as well as in the liquid state. The results show that the compounds appearing as rigorously planar in the solid present instead a twisted conformation in the melt. The solid versus melt issue strongly suggests that the reasons for planarity are to be found in the packing restraints. A `rule of thumb' is suggested for the design of biphenyls with different molecular conformations, based on the selection of the OR substituent.
中文翻译:

3,3',4,4'-取代的联苯的合成及晶体学,光谱学和计算表征:OR取代基对环内扭转角的影响。
本文介绍了在3、3',4和4'位被各种R取代的一系列联苯的结构的合成,表征和研究(使用单晶X射线衍射,拉曼散射,量子力学计算)(R=甲基,乙酰基,己基)基团通过氧原子连接至联苯核。在固态和液态下都已广泛研究了分子构象,特别是芳环之间的扭转角。结果表明,化合物在固体中表现为严格平面,而不是在熔体中呈扭曲构象。该固体与熔体强烈建议在包装约束中找到平面性的原因。建议根据O R取代基的选择,设计具有不同分子构象的联苯的“经验法则” 。
更新日期:2020-05-18
中文翻译:

3,3',4,4'-取代的联苯的合成及晶体学,光谱学和计算表征:OR取代基对环内扭转角的影响。
本文介绍了在3、3',4和4'位被各种R取代的一系列联苯的结构的合成,表征和研究(使用单晶X射线衍射,拉曼散射,量子力学计算)(R=甲基,乙酰基,己基)基团通过氧原子连接至联苯核。在固态和液态下都已广泛研究了分子构象,特别是芳环之间的扭转角。结果表明,化合物在固体中表现为严格平面,而不是在熔体中呈扭曲构象。该固体与熔体强烈建议在包装约束中找到平面性的原因。建议根据O R取代基的选择,设计具有不同分子构象的联苯的“经验法则” 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号