当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of (−)-protulactone A
Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-17 , DOI: 10.1016/j.tet.2020.131290 Qingwei Lv , Caizhu Chang , Yong Li , Yuguo Du , Jun Liu
中文翻译:

(-)-丙内酯A的立体选择性合成
更新日期:2020-05-17
Tetrahedron ( IF 2.1 ) Pub Date : 2020-05-17 , DOI: 10.1016/j.tet.2020.131290 Qingwei Lv , Caizhu Chang , Yong Li , Yuguo Du , Jun Liu
|
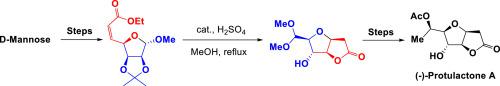
An efficient stereoselective synthesis of (−)-protulactone A was established in 9 steps and 14.7% overall yields using d-mannose as starting material. The key steps of our synthesis involved one-pot deprotection/lactonization/Michael addition reaction/acetalization and 1, 3-chelated controlled stereoselective addition.
中文翻译:

(-)-丙内酯A的立体选择性合成
以d-甘露糖为起始原料,通过9个步骤建立了有效的立体选择性合成(-)-丙内酯A,总产率为14.7%。我们合成的关键步骤涉及一锅脱保护/内酰胺化/迈克尔加成反应/缩醛化和1,3螯合的受控立体选择性加成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号