Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2020-05-18 , DOI: 10.1016/j.jmgm.2020.107640 Swapnil S Deshpande 1 , Dipali B Potekar 2 , Pradip B Shelke 3 , Mrinalini D Deshpande 1
|
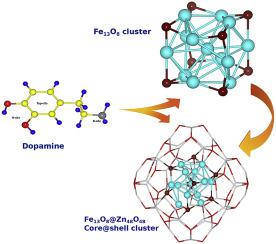
In this study, we modelled the interaction of Fe13O8 and Fe13O8@Zn48O48 ([email protected]) cluster with a biologically active dopamine molecule using density functional theory. First, the electronic, magnetic and optical properties of [email protected], Fe13O8@Zn48O48 cluster investigated and compared with isolated Fe13O8 and Zn48O48 clusters. Fe13O8@Zn48O48 cluster is found to be energetically stable. For Fe13O8 and Fe13O8@Zn48O48 clusters have the net magnetic moment 42 . The decrease in HOMO-LUMO gap of [email protected] cluster as compared to that of isolated clusters reflects the higher reactivity. The results of the site dependent interaction of Fe13O8 and Fe13O8@Zn48O48 clusters with dopamine molecule are presented. The interaction strength is determined in terms of the cluster-dopamine complex binding energy and found to be enhanced for [email protected] cluster than the Fe13O8. Furthermore, the calculated results predict that in presence of dopamine, the magnetic moment of Fe13O8 and Fe13O8@Zn48O48 cluster remains unaffected. The analysis of optical spectra of [email protected] indicates the obvious red shift compared to Zn48O48 clusters. The optical spectra of Fe13O8@Zn48O48-dopamine shows the higher oscillator strength as compared to that of Fe13O8-dopamine complex. Fe13O8-dopamine complex gives rise to more quenched oscillator strengths as compared to that of bare iron oxide cluster. These results indicate interesting magneto-optical behaviour, which can be useful for biomedical applications.
中文翻译:

Fe13O8 @ Zn48O48团簇与多巴胺相互作用的理论研究:磁性和光学性质。
在这项研究中,我们使用密度泛函理论模拟了Fe 13 O 8和Fe 13 O 8 @Zn 48 O 48([电子邮件保护])簇与具有生物活性的多巴胺分子的相互作用。首先,研究了[受电子邮件保护的] Fe 13 O 8 @Zn 48 O 48团簇的电子,磁和光学性质,并与孤立的Fe 13 O 8和Zn 48 O 48团簇进行了比较。铁13 O 8 @锌48 O 48发现该簇是能量稳定的。对于Fe 13 O 8和Fe 13 O 8 @Zn 48 O 48簇具有净磁矩42。与孤立的群集相比,[受电子邮件保护的]群集的HOMO-LUMO缺口的减少反映了更高的反应性。给出了Fe 13 O 8和Fe 13 O 8 @Zn 48 O 48团簇与多巴胺分子的位置依赖性相互作用的结果。相互作用强度取决于簇-多巴胺络合物的结合能,并且发现对于[电子邮件保护的]簇,其相互作用强度高于Fe 13 O 8。此外,计算结果预测,在多巴胺的存在下,Fe 13 O 8和Fe 13 O 8的磁矩@Zn 48 O 48团簇不受影响。对[受电子邮件保护]的光谱分析表明,与Zn 48 O 48团簇相比,红移明显。与Fe 13 O 8-多巴胺络合物相比,Fe 13 O 8 @Zn 48 O 48-多巴胺的光谱显示出更高的振荡强度。与裸氧化铁簇相比,Fe 13 O 8-多巴胺络合物具有更高的淬灭振子强度。这些结果表明有趣的磁光行为,可用于生物医学应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号