当前位置:
X-MOL 学术
›
Drug Metab. Pharmacokinet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel variants in outer protein surface of flavin-containing monooxygenase 3 found in an Argentinian case with impaired capacity for trimethylamine N-oxygenation
Drug Metabolism and Pharmacokinetics ( IF 2.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.dmpk.2020.05.003 Leonardo Dionisio 1 , Makiko Shimizu 2 , Sofia Stupniki 1 , Saki Oyama 2 , Eugenio Aztiria 1 , Maximiliano Alda 3 , Hiroshi Yamazaki 2 , Guillermo Spitzmaul 1
Drug Metabolism and Pharmacokinetics ( IF 2.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.dmpk.2020.05.003 Leonardo Dionisio 1 , Makiko Shimizu 2 , Sofia Stupniki 1 , Saki Oyama 2 , Eugenio Aztiria 1 , Maximiliano Alda 3 , Hiroshi Yamazaki 2 , Guillermo Spitzmaul 1
Affiliation
|
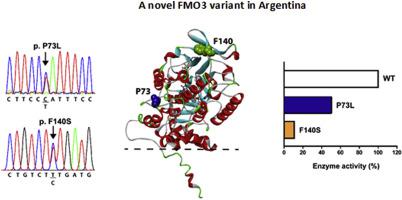
Flavin-containing monooxygenase 3 (FMO3) is a polymorphic drug metabolizing enzyme associated with the genetic disorder trimethylaminuria. We phenotyped a white Argentinian 11-year-old girl by medical sensory evaluation. After pedigree analysis with her brother and parents, this proband showed to harbor a new allele p.(P73L; E158K; E308G) FMO3 in trans configuration with the second new one p.(F140S) FMO3. Recombinant FMO3 proteins of the wild-type and the novel two variants underwent kinetic analyses of their trimethylamine N-oxygenation activities. P73L; E158K; E308G and F140S FMO3 proteins exhibited moderately and severely decreased trimethylamine N-oxygenation capacities (~50% and ~10% of wild-type FMO3, respectively). Amino acids P73 and F140 were located on the outer surface region in a crystallographic structure recently reported of a FMO3 analog. Changes in these positions would indirectly impact on key FAD-binding residues. This is the first report and characterization of a patient of fish odor syndrome caused by genetic aberrations leading to impaired FMO3-dependent N-oxygenation of trimethylamine found in the Argentinian population. We found novel structural determinants of FAD-binding domains, expanding the list of known disease-causing mutations of FMO3. Our results suggest that individuals homozygous for any of these new variants would develop a severe form of this disorder.
中文翻译:

在阿根廷病例中发现的含黄素单加氧酶 3 的外蛋白表面的新变体,其三甲胺 N-氧化能力受损
含黄素的单加氧酶 3 (FMO3) 是一种多态性药物代谢酶,与遗传性疾病三甲基氨基尿症相关。我们通过医学感官评估对一名 11 岁的阿根廷白人女孩进行了表型分析。在与她的兄弟和父母进行谱系分析后,该先证者显示携带一个新的等位基因 p.(P73L; E158K; E308G) FMO3 与第二个新的 p.(F140S) FMO3 处于反式构型。野生型和新的两个变体的重组 FMO3 蛋白对其三甲胺 N-氧化活性进行了动力学分析。P73L; E158K;E308G 和 F140S FMO3 蛋白表现出中度和严重降低的三甲胺 N 氧化能力(分别为野生型 FMO3 的约 50% 和约 10%)。氨基酸 P73 和 F140 位于最近报道的 FMO3 类似物晶体结构的外表面区域。这些位置的变化将间接影响关键的 FAD 结合残基。这是对阿根廷人群中发现的三甲胺的 FMO3 依赖性 N-氧化受损的遗传畸变引起的鱼味综合征患者的首次报告和表征。我们发现了 FAD 结合域的新结构决定因素,扩大了 FMO3 已知致病突变的列表。我们的结果表明,任何这些新变异的纯合子个体都会发展为严重的这种疾病。这是对阿根廷人群中发现的三甲胺的 FMO3 依赖性 N-氧化受损的遗传畸变引起的鱼味综合征患者的首次报告和表征。我们发现了 FAD 结合域的新结构决定因素,扩大了 FMO3 已知致病突变的列表。我们的结果表明,任何这些新变异的纯合子个体都会发展为严重的这种疾病。这是对阿根廷人群中发现的三甲胺的 FMO3 依赖性 N-氧化受损的遗传畸变引起的鱼味综合征患者的首次报告和表征。我们发现了 FAD 结合域的新结构决定因素,扩大了 FMO3 已知致病突变的列表。我们的结果表明,任何这些新变异的纯合子个体都会发展为严重的这种疾病。
更新日期:2020-08-01
中文翻译:

在阿根廷病例中发现的含黄素单加氧酶 3 的外蛋白表面的新变体,其三甲胺 N-氧化能力受损
含黄素的单加氧酶 3 (FMO3) 是一种多态性药物代谢酶,与遗传性疾病三甲基氨基尿症相关。我们通过医学感官评估对一名 11 岁的阿根廷白人女孩进行了表型分析。在与她的兄弟和父母进行谱系分析后,该先证者显示携带一个新的等位基因 p.(P73L; E158K; E308G) FMO3 与第二个新的 p.(F140S) FMO3 处于反式构型。野生型和新的两个变体的重组 FMO3 蛋白对其三甲胺 N-氧化活性进行了动力学分析。P73L; E158K;E308G 和 F140S FMO3 蛋白表现出中度和严重降低的三甲胺 N 氧化能力(分别为野生型 FMO3 的约 50% 和约 10%)。氨基酸 P73 和 F140 位于最近报道的 FMO3 类似物晶体结构的外表面区域。这些位置的变化将间接影响关键的 FAD 结合残基。这是对阿根廷人群中发现的三甲胺的 FMO3 依赖性 N-氧化受损的遗传畸变引起的鱼味综合征患者的首次报告和表征。我们发现了 FAD 结合域的新结构决定因素,扩大了 FMO3 已知致病突变的列表。我们的结果表明,任何这些新变异的纯合子个体都会发展为严重的这种疾病。这是对阿根廷人群中发现的三甲胺的 FMO3 依赖性 N-氧化受损的遗传畸变引起的鱼味综合征患者的首次报告和表征。我们发现了 FAD 结合域的新结构决定因素,扩大了 FMO3 已知致病突变的列表。我们的结果表明,任何这些新变异的纯合子个体都会发展为严重的这种疾病。这是对阿根廷人群中发现的三甲胺的 FMO3 依赖性 N-氧化受损的遗传畸变引起的鱼味综合征患者的首次报告和表征。我们发现了 FAD 结合域的新结构决定因素,扩大了 FMO3 已知致病突变的列表。我们的结果表明,任何这些新变异的纯合子个体都会发展为严重的这种疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号