Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neuroglial transmitophagy and Parkinson's disease.
Glia ( IF 5.4 ) Pub Date : 2020-05-16 , DOI: 10.1002/glia.23839 Ingrid Morales 1, 2 , Alberto Sanchez 1 , Ricardo Puertas-Avendaño 1 , Clara Rodriguez-Sabate 2 , Adrian Perez-Barreto 1 , Manuel Rodriguez 1, 2
Glia ( IF 5.4 ) Pub Date : 2020-05-16 , DOI: 10.1002/glia.23839 Ingrid Morales 1, 2 , Alberto Sanchez 1 , Ricardo Puertas-Avendaño 1 , Clara Rodriguez-Sabate 2 , Adrian Perez-Barreto 1 , Manuel Rodriguez 1, 2
Affiliation
|
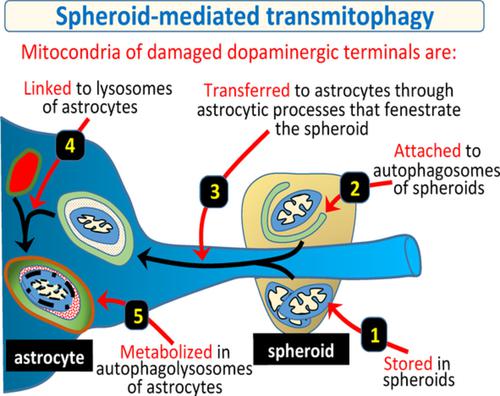
Mitophagy is essential for the health of dopaminergic neurons because mitochondrial damage is a keystone of Parkinson's disease. The aim of the present work was to study the degradation of mitochondria in the degenerating dopaminergic synapse. Adult Sprague–Dawley rats and YFP‐Mito‐DAn mice with fluorescent mitochondria in dopaminergic neurons were injected in the lateral ventricles with 6‐hydroxydopamine, a toxic that inhibits the mitochondrial chain of dopaminergic neurons and blockades the axonal transport. Dopaminergic terminals closest to the lateral ventricle showed an axonal fragmentation and an accumulation of damaged mitochondria in 2–9 μ saccular structures (spheroids). Damaged mitochondria accumulated in spheroids initiated (showing high Pink1, parkin, ubiquitin, p‐S65‐Ubi, AMBRA1, and BCL2L13 immunoreactivity and developing autophagosomes) but did not complete (mitochondria were not polyubiquitinated, autophagosomes had no STX17, and no lysosomes were found in spheroids) the mitophagy process. Then, spheroids were penetrated by astrocytic processes and DAergic mitochondria were transferred to astrocytes where they were polyubiquitinated (UbiK63+) and linked to mature autophagosomes (STX17+) which became autophagolysosomes (Lamp1/Lamp2 which co‐localized with LC3). Present data provide evidence that the mitophagy of degenerating dopaminergic terminals starts in the dopaminergic spheroids and finishes in the surrounding astrocytes (spheroid‐mediated transmitophagy). The neuron‐astrocyte transmitophagy could be critical for preventing the release of damaged mitochondria to the extracellular medium and the neuro‐inflammatory activity which characterizes Parkinson's disease.
中文翻译:

神经胶质细胞自噬和帕金森病。
线粒体自噬对多巴胺能神经元的健康至关重要,因为线粒体损伤是帕金森病的关键。目前工作的目的是研究退化的多巴胺能突触中线粒体的降解。在多巴胺能神经元中具有荧光线粒体的成年 Sprague-Dawley 大鼠和 YFP-Mito-DAn 小鼠在侧脑室注射 6-羟基多巴胺,这是一种抑制多巴胺能神经元线粒体链并阻断轴突运输的毒性物质。最靠近侧脑室的多巴胺能末梢显示轴突断裂和受损线粒体在 2-9 μ 囊状结构(球体)中的积累。损伤的线粒体积累在启动的球体中(显示高 Pink1、parkin、泛素、p-S65-Ubi、AMBRA1、和 BCL2L13 免疫反应性和发展自噬体)但没有完成(线粒体没有被多泛素化,自噬体没有 STX17,在球体中没有发现溶酶体)线粒体自噬过程。然后,球体被星形胶质细胞过程穿透,DAergic 线粒体被转移到星形胶质细胞,在那里它们被多泛素化 (UbiK63+) 并连接到成熟的自噬体 (STX17+),后者成为自噬溶酶体 (Lamp1/Lamp2 与 LC3 共定位)。目前的数据提供证据表明,退化的多巴胺能末端的线粒体自噬始于多巴胺能球体,并在周围的星形胶质细胞中结束。球体被星形胶质细胞过程穿透,DAergic 线粒体被转移到星形胶质细胞,在那里它们被多泛素化 (UbiK63+) 并连接到成熟的自噬体 (STX17+),后者成为自噬溶酶体 (Lamp1/Lamp2 与 LC3 共定位)。目前的数据提供证据表明,退化的多巴胺能末端的线粒体自噬始于多巴胺能球体,并在周围的星形胶质细胞中结束。球体被星形胶质细胞过程穿透,DAergic 线粒体被转移到星形胶质细胞,在那里它们被多泛素化 (UbiK63+) 并连接到成熟的自噬体 (STX17+),后者成为自噬溶酶体 (Lamp1/Lamp2 与 LC3 共定位)。目前的数据提供证据表明,退化的多巴胺能末端的线粒体自噬始于多巴胺能球体,并在周围的星形胶质细胞中结束。球体介导的自噬)。神经元-星形胶质细胞的自噬对于防止受损的线粒体释放到细胞外介质和帕金森病的神经炎症活动至关重要。
更新日期:2020-05-16
中文翻译:

神经胶质细胞自噬和帕金森病。
线粒体自噬对多巴胺能神经元的健康至关重要,因为线粒体损伤是帕金森病的关键。目前工作的目的是研究退化的多巴胺能突触中线粒体的降解。在多巴胺能神经元中具有荧光线粒体的成年 Sprague-Dawley 大鼠和 YFP-Mito-DAn 小鼠在侧脑室注射 6-羟基多巴胺,这是一种抑制多巴胺能神经元线粒体链并阻断轴突运输的毒性物质。最靠近侧脑室的多巴胺能末梢显示轴突断裂和受损线粒体在 2-9 μ 囊状结构(球体)中的积累。损伤的线粒体积累在启动的球体中(显示高 Pink1、parkin、泛素、p-S65-Ubi、AMBRA1、和 BCL2L13 免疫反应性和发展自噬体)但没有完成(线粒体没有被多泛素化,自噬体没有 STX17,在球体中没有发现溶酶体)线粒体自噬过程。然后,球体被星形胶质细胞过程穿透,DAergic 线粒体被转移到星形胶质细胞,在那里它们被多泛素化 (UbiK63+) 并连接到成熟的自噬体 (STX17+),后者成为自噬溶酶体 (Lamp1/Lamp2 与 LC3 共定位)。目前的数据提供证据表明,退化的多巴胺能末端的线粒体自噬始于多巴胺能球体,并在周围的星形胶质细胞中结束。球体被星形胶质细胞过程穿透,DAergic 线粒体被转移到星形胶质细胞,在那里它们被多泛素化 (UbiK63+) 并连接到成熟的自噬体 (STX17+),后者成为自噬溶酶体 (Lamp1/Lamp2 与 LC3 共定位)。目前的数据提供证据表明,退化的多巴胺能末端的线粒体自噬始于多巴胺能球体,并在周围的星形胶质细胞中结束。球体被星形胶质细胞过程穿透,DAergic 线粒体被转移到星形胶质细胞,在那里它们被多泛素化 (UbiK63+) 并连接到成熟的自噬体 (STX17+),后者成为自噬溶酶体 (Lamp1/Lamp2 与 LC3 共定位)。目前的数据提供证据表明,退化的多巴胺能末端的线粒体自噬始于多巴胺能球体,并在周围的星形胶质细胞中结束。球体介导的自噬)。神经元-星形胶质细胞的自噬对于防止受损的线粒体释放到细胞外介质和帕金森病的神经炎症活动至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号