当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-bond 1 J (15 N,H) coupling constants at sp2 hybridized nitrogen of Schiff bases, enaminones and similar compounds. A theoretical study
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-06-17 , DOI: 10.1002/mrc.5052 Poul Erik Hansen 1 , Bahjat A Saeed 2 , Rita S Rutu 3 , Teobald Kupka 4
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-06-17 , DOI: 10.1002/mrc.5052 Poul Erik Hansen 1 , Bahjat A Saeed 2 , Rita S Rutu 3 , Teobald Kupka 4
Affiliation
|
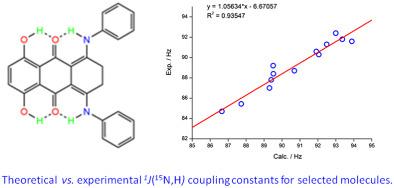
1J(15N,H) coupling constants for enaminones and NH‐forms of intramolecularly hydrogen‐bonded Schiff bases as model compounds for sp2‐hybridized nitrogen atoms are evaluated using density functional theory (DFT) to find the optimal functionals and basis sets. Ammonia is used as a test molecule and its one‐bond coupling constant is compared with experiment. A methylamine Schiff base of a truncated molecule of gossypol is used for checking the performance of selected B3LYP, O3LYP, PBE, BHandH, and APFD density functionals and standard, modified, and dedicated basis sets for coupling constants. Both in vacuum and in chloroform, modeled by the simple continuum model of solvent, the modified basis sets predict significantly better the 1J(15N,H) value in ammonia and in the methylamine Schiff base of a truncated molecule of gossypol than the standard basis sets. This procure is then used on a broad set of intramolecularly hydrogen‐bonded molecules, and a good correlation between calculated and experimental one‐bond NH coupling constants is obtained. The 1J(15N,H) couplings are slightly overestimated. The calculated data show for hydrogen‐bonded NH interatomic distances that the calculated values depend on the NH bond lengths. The shorter the bond lengths, the larger the 1J(15N,H). A useful correlation between 1J(15N,H) and NH bond length is derived that enables realistic predictions of one‐bond NH coupling constants. The calculations reproduce experimentally observed trends for the studied molecules.
中文翻译:

席夫碱、烯胺酮和类似化合物的 sp2 杂化氮处的单键 1 J (15 N,H) 偶联常数。理论研究
使用密度泛函理论 (DFT) 评估烯胺酮和分子内氢键希夫碱的 NH 形式作为 sp2 杂化氮原子模型化合物的 1J(15N,H) 偶联常数,以找到最佳泛函和基组。以氨为试验分子,并与实验比较其单键偶联常数。棉酚截短分子的甲胺席夫碱用于检查所选 B3LYP、O3LYP、PBE、BHandH 和 APFD 密度泛函的性能以及耦合常数的标准、修改和专用基组。在真空和氯仿中,通过溶剂的简单连续模型建模,修改后的基组对 1J(15N, H) 棉酚截短分子在氨和甲胺席夫碱中的值,高于标准基组。然后将此采购用于一系列广泛的分子内氢键分子,并获得计算和实验单键 NH 耦合常数之间的良好相关性。1J(15N,H) 联轴器被稍微高估了。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。然后将此采购用于广泛的分子内氢键分子集,并获得计算和实验单键 NH 耦合常数之间的良好相关性。1J(15N,H) 联轴器被稍微高估了。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。然后将此采购用于一系列广泛的分子内氢键分子,并获得计算和实验单键 NH 耦合常数之间的良好相关性。1J(15N,H) 联轴器被稍微高估了。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。
更新日期:2020-06-17
中文翻译:

席夫碱、烯胺酮和类似化合物的 sp2 杂化氮处的单键 1 J (15 N,H) 偶联常数。理论研究
使用密度泛函理论 (DFT) 评估烯胺酮和分子内氢键希夫碱的 NH 形式作为 sp2 杂化氮原子模型化合物的 1J(15N,H) 偶联常数,以找到最佳泛函和基组。以氨为试验分子,并与实验比较其单键偶联常数。棉酚截短分子的甲胺席夫碱用于检查所选 B3LYP、O3LYP、PBE、BHandH 和 APFD 密度泛函的性能以及耦合常数的标准、修改和专用基组。在真空和氯仿中,通过溶剂的简单连续模型建模,修改后的基组对 1J(15N, H) 棉酚截短分子在氨和甲胺席夫碱中的值,高于标准基组。然后将此采购用于一系列广泛的分子内氢键分子,并获得计算和实验单键 NH 耦合常数之间的良好相关性。1J(15N,H) 联轴器被稍微高估了。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。然后将此采购用于广泛的分子内氢键分子集,并获得计算和实验单键 NH 耦合常数之间的良好相关性。1J(15N,H) 联轴器被稍微高估了。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。然后将此采购用于一系列广泛的分子内氢键分子,并获得计算和实验单键 NH 耦合常数之间的良好相关性。1J(15N,H) 联轴器被稍微高估了。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。计算数据表明,对于氢键合的 NH 原子间距离,计算值取决于 NH 键长。键长越短,1J(15N,H)越大。推导出 1J(15N,H) 和 NH 键长之间的有用相关性,可以对单键 NH 耦合常数进行实际预测。计算再现了所研究分子的实验观察趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号