当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Catalytic Conversion of Ethanol to C5+ Ketones: Role of Pd-Zn Alloy on Catalytic Activity and Stability.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-15 , DOI: 10.1002/anie.202005256 Senthil Subramaniam 1, 2 , Mond F Guo 1, 2 , Tanmayi Bathena 3 , Michel Gray 1 , Xiao Zhang 1, 2 , Abraham Martinez 1 , Libor Kovarik 1 , Konstantinos A Goulas 3 , Karthikeyan K Ramasamy 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-15 , DOI: 10.1002/anie.202005256 Senthil Subramaniam 1, 2 , Mond F Guo 1, 2 , Tanmayi Bathena 3 , Michel Gray 1 , Xiao Zhang 1, 2 , Abraham Martinez 1 , Libor Kovarik 1 , Konstantinos A Goulas 3 , Karthikeyan K Ramasamy 1
Affiliation
|
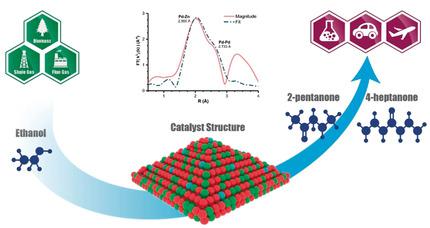
Ethanol can be used as a platform molecule for synthesizing valuable chemicals and fuel precursors. Direct synthesis of C5+ ketones, building blocks for lubricants and hydrocarbon fuels, from ethanol was achieved over a stable Pd‐promoted ZnO‐ZrO2 catalyst. The sequence of reaction steps involved in the C5+ ketone formation from ethanol was determined. The key reaction steps were found to be the in situ generation of the acetone intermediate and the cross‐aldol condensation between the reaction intermediates acetaldehyde and acetone. The formation of a Pd–Zn alloy in situ was identified to be the critical factor in maintaining high yield to the C5+ ketones and the stability of the catalyst. A yield of >70 % to C5+ ketones was achieved over a 0.1 % Pd‐ZnO‐ZrO2 mixed oxide catalyst, and the catalyst was demonstrated to be stable beyond 2000 hours on stream without any catalyst deactivation.
中文翻译:

乙醇直接催化转化为C5 +酮:Pd-Zn合金对催化活性和稳定性的作用。
乙醇可用作合成有价值的化学物质和燃料前体的平台分子。在稳定的Pd促进的ZnO-ZrO 2催化剂上,可以从乙醇中直接合成C 5+酮,润滑剂和烃类燃料的基础材料。确定了由乙醇形成C 5+酮所涉及的反应步骤的顺序。发现关键的反应步骤是丙酮中间体的原位生成以及反应中间体乙醛和丙酮之间的交叉羟醛缩合。已确定原位形成Pd-Zn合金是保持C 5+酮的高收率和催化剂稳定性的关键因素。C 5+的收率> 70%在0.1%的Pd-ZnO-ZrO 2混合氧化物催化剂上获得了酮,并且该催化剂在运行过程中经过2000小时稳定,并且没有任何催化剂失活。
更新日期:2020-05-15
中文翻译:

乙醇直接催化转化为C5 +酮:Pd-Zn合金对催化活性和稳定性的作用。
乙醇可用作合成有价值的化学物质和燃料前体的平台分子。在稳定的Pd促进的ZnO-ZrO 2催化剂上,可以从乙醇中直接合成C 5+酮,润滑剂和烃类燃料的基础材料。确定了由乙醇形成C 5+酮所涉及的反应步骤的顺序。发现关键的反应步骤是丙酮中间体的原位生成以及反应中间体乙醛和丙酮之间的交叉羟醛缩合。已确定原位形成Pd-Zn合金是保持C 5+酮的高收率和催化剂稳定性的关键因素。C 5+的收率> 70%在0.1%的Pd-ZnO-ZrO 2混合氧化物催化剂上获得了酮,并且该催化剂在运行过程中经过2000小时稳定,并且没有任何催化剂失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号