Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-05-16 , DOI: 10.1016/j.jfluchem.2020.109566 Mousa Soleymani
|
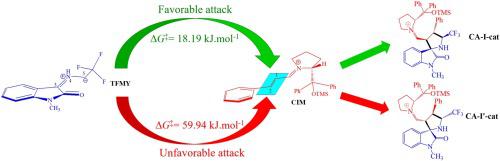
Organocatalytic asymmetric [3 + 2] cycloaddition reaction of 3-(2,2,2-trifluoroethylimino)-1-methylindolin-2-one ylide TFMY with the cinamaldehyde CIA and with the corresponding iminium ion CIM, is studied using molecular electron density theory. This reaction which leads to the formation of certain CF3-containing spiro[pyrrolidin-3,2′-oxindoles] has been explored experimentally by Ma and coworkers. Analysis of the global CDFT indices revealed that CIA as well as CIM show a more negative value of electronic chemical potential in comparison to TFMY. In addition, it was found that by conversion of CIA to the corresponding iminium ion CIM, the global CDFT indices change significantly. In order to study the regioselectivity, the local reactivity indices based on the Parr functions were calculated and the results showed an excellent agreement with the experimental outcomes. The diastereoselectivity of the reaction was investigated by PES analysis and a good agreement was observed with the experimental results. By calculation of the molecular electrostatic potential (MEP) map for transition states, it was found that the electrostatic forces between two fragments can explain the observed diastereoselectivity. The enantioselectivity of the reaction was also investigated with an emphasis on the effect of the chiral organocatalyst (diphenylprolinol silyl ether 5) on the [3 + 2] cycloaddition reaction. The PES analysis showed that the bulky group located on the organocatalyst in the iminium ion CIM determines the direction of the cycloaddition. Indeed, by conversion of CIA to CIM, the reactivity, regioselectivity and enantioselectivity are significantly improved in reaction. The molecular mechanism of the asymmetric reaction was investigated by the IRC and QTAIM analyses and the results suggested a two-stage one-step mechanism for the reaction. Finally, by calculation of the rate as well as equilibrium constants for deprotonation of TFMY precursor, it was found that the CF3 group as an electron-withdrawing one plays an important role in the production of TFMY.
中文翻译:

通过有机催化[3 + 2]环加成反应合成含CF 3的螺[吡咯烷-3,2'-羟吲哚]的区域,非对映和对映选择性:分子电子密度理论研究
利用分子电子密度理论研究了3-(2,2,2-三氟乙基亚氨基)-1-甲基吲哚-2-酮内酯TFMY与肉桂醛CIA以及相应的亚胺离子CIM的有机催化不对称[3 + 2]环加成反应。。Ma及其同事已经通过实验探索了导致形成某些含CF 3的螺[吡咯烷基3,2'-羟吲哚]的反应。对全球CDFT指数的分析显示,与TFMY相比,CIA和CIM表现出更大的电子化学势负值。另外,发现通过CIA的转换与相应的亚胺离子CIM相比,全球CDFT指数发生了显着变化。为了研究区域选择性,计算了基于Parr函数的局部反应性指数,结果与实验结果非常吻合。通过PES分析研究了反应的非对映选择性,并与实验结果相吻合。通过计算过渡态的分子静电势(MEP)图,发现两个片段之间的静电力可以解释观察到的非对映选择性。还研究了反应的对映选择性,重点是手性有机催化剂(二苯基脯氨醇甲硅烷基醚5)在[3 + 2]环加成反应上。PES分析表明,亚胺离子CIM中位于有机催化剂上的庞大基团决定了环加成的方向。实际上,通过将CIA转化为CIM,反应中的反应性,区域选择性和对映选择性显着提高。通过IRC和QTAIM分析研究了不对称反应的分子机理,结果表明该反应是两阶段的一步反应机理。最后,通过计算TFMY前体去质子化的速率和平衡常数,发现CF 3作为吸电子基团在TFMY的生产中起重要作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号