Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-05-16 , DOI: 10.1016/j.actbio.2020.04.048 Dong Hwa Kim 1 , Julianne Huegel 1 , Brittany L Taylor 1 , Courtney A Nuss 1 , Stephanie N Weiss 1 , Louis J Soslowsky 2 , Robert L Mauck 2 , Andrew F Kuntz 1
|
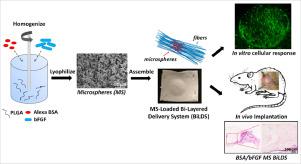
Many drug delivery systems rely on degradation or dissolution of the carrier material to regulate release. In cases where mechanical support is required during regeneration, this necessitates composite systems in which the mechanics of the implant are decoupled from the drug release profile. To address this need, we developed a system in which microspheres (MS) were sequestered in a defined location between two nanofibrous layers. This bilayer delivery system (BiLDS) enables simultaneous structural support and decoupled release profiles. To test this new system, PLGA (poly-lactide-co-glycolic acid) microspheres were prepared using a water-in-oil-in-water (w/o/w) emulsion technique and incorporated Alexa Fluor-tagged bovine serum albumin (BSA) and basic fibroblast growth factor (bFGF). These MS were secured in a defined pocket between two polycaprolactone (PCL) nanofibrous scaffolds, where the layered scaffolds provide a template for new tissue formation while enabling independent and local release from the co-delivered MS. Scanning electron microscopy (SEM) images showed that the assembled BiLDS could localize and retain MS in the central pocket that was surrounded by a continuous seal formed along the margin. Cell viability and proliferation assays showed enhanced cell activity when exposed to BiLDS containing Alexa Fluor-BSA/bFGF-loaded MS, both in vitro and in vivo. MS delivered via the BiLDS system persisted in a localized area after subcutaneous implantation for at least 4 weeks, and bFGF release increased colonization of the implant. These data establish the BiLDS technology as a sustained in vivo drug delivery platform that can localize protein and other growth factor release to a surgical site while providing a structural template for new tissue formation.
Statement of Significance
Localized and controlled delivery systems for the sustained release of drugs are essential. Many strategies have been developed for this purpose, but most rely on degradation (and loss of material properties) for delivery. Here, we developed a bilayer delivery system (BiLDS) that decouples the physical properties of a scaffold from its delivery kinetics. For this, biodegradable PLGA microspheres were sequestered within a central pocket of a slowly degrading nanofibrous bilayer. Using this device, we show enhanced cell activity with FGF delivery from the BiLDS both in vitro and in vivo. These data support that BiLDS can localize sustained protein and biofactor delivery to a surgical site while also serving as a mechanical scaffold for tissue repair and regeneration.
中文翻译:

载有FGF的微球双层递送系统的生物相容性和生物活性。
许多药物递送系统依靠载体材料的降解或溶解来调节释放。在再生过程中需要机械支撑的情况下,这需要复合系统,其中植入物的力学与药物释放曲线不相关。为了满足这一需求,我们开发了一种系统,其中微球(MS)被隔离在两个纳米纤维层之间的指定位置。这种双层输送系统(BiLDS)可以同时进行结构支撑和脱钩的释放曲线。为了测试该新系统,使用水包油包水(w / o / w)乳液技术制备了PLGA(聚丙交酯-乙醇酸共聚物)微球,并掺入了Alexa Fluor标记的牛血清白蛋白( BSA)和碱性成纤维细胞生长因子(bFGF)。这些MS被固定在两个聚己内酯(PCL)纳米纤维支架之间的明确口袋中,其中分层支架为新组织的形成提供模板,同时能够从共同提供的MS中独立和局部释放。扫描电子显微镜(SEM)图像显示,组装的BiLDS可以将MS定位并保留在中央囊袋中,该中央囊袋被沿边缘形成的连续密封件包围。当暴露于含有Alexa Fluor-BSA / bFGF负载MS的BiLDS时,无论是在体内还是体外,细胞活力和增殖测定均显示出增强的细胞活性。皮下植入后,通过BiLDS系统递送的MS持续存在于局部区域,并且bFGF释放增加了植入物的定植。
重要声明
持续释放药物的本地化和受控递送系统至关重要。为此目的已经开发了许多策略,但是大多数策略依赖于降解(和材料特性的损失)来进行传递。在这里,我们开发了一种双层输送系统(BiLDS),该系统将支架的物理特性与其输送动力学脱钩。为此,将可生物降解的PLGA微球固定在缓慢降解的纳米纤维双层的中央口袋中。使用此设备,我们展示了从BiLDS体内外递送FGF所增强的细胞活性。这些数据支持BiLDS可以将持续的蛋白质和生物因子输送到手术部位,同时还可以用作组织修复和再生的机械支架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号