当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Validating Signal Transducer and Activator of Transcription (STAT) Protein-Inhibitor Interactions Using Biochemical and Cellular Thermal Shift Assays.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-05-15 , DOI: 10.1021/acschembio.0c00046 Sanaz Attarha 1, 2 , Anja Reithmeier 2, 3, 4 , Sander Busker 3 , Matthieu Desroses 1, 2 , Brent D G Page 1, 2, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-05-15 , DOI: 10.1021/acschembio.0c00046 Sanaz Attarha 1, 2 , Anja Reithmeier 2, 3, 4 , Sander Busker 3 , Matthieu Desroses 1, 2 , Brent D G Page 1, 2, 5
Affiliation
|
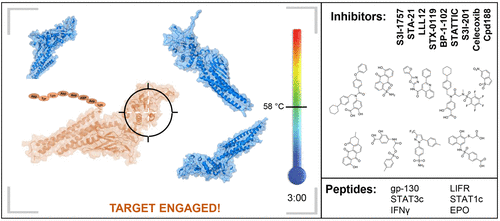
Signal transducer and activator of transcription (STAT) proteins have important biological functions; however, deregulation of STAT signaling is a driving force behind the onset and progression of inflammatory diseases and cancer. While their biological roles suggest that STAT proteins would be valuable targets for developing therapeutic agents, STAT proteins are notoriously difficult to inhibit using small drug-like molecules, as they do not have a distinct inhibitor binding site. Despite this, a multitude of small-molecule STAT inhibitors have been proposed, primarily focusing on inhibiting STAT3 protein to generate novel cancer therapies. Demonstrating that inhibitors bind to their targets in cells has historically been a very challenging task. With the advent of modern target engagement techniques, such as the cellular thermal shift assay (CETSA), interactions between experimental compounds and their biological targets can be detected with relative ease. To investigate interactions between STAT proteins and inhibitors, we herein developed STAT CETSAs and evaluated known STAT3 inhibitors for their ability to engage STAT proteins in biological settings. While potent binding was detected between STAT proteins and peptidic STAT inhibitors, small-molecule inhibitors elicited variable responses, most of which failed to stabilize STAT3 proteins in cells and cell lysates. The described STAT thermal stability assays represent valuable tools for evaluating proposed STAT inhibitors.
中文翻译:

使用生化和细胞热位移分析验证信号转导和转录激活剂(STAT)蛋白抑制剂的相互作用。
信号转导子和转录激活子(STAT)蛋白具有重要的生物学功能。然而,STAT信号转导的失调是炎性疾病和癌症的发作和发展的驱动力。尽管它们的生物学作用表明STAT蛋白将成为开发治疗剂的重要靶标,但是众所周知,STAT蛋白很难用类药物小分子抑制,因为它们没有明显的抑制剂结合位点。尽管如此,已经提出了许多小分子STAT抑制剂,主要集中在抑制STAT3蛋白以产生新的癌症疗法上。过去,证明抑制剂与细胞中的靶标结合一直是一项非常艰巨的任务。随着现代目标交战技术的出现,例如细胞热位移分析(CETSA),可以相对轻松地检测实验化合物与其生物学靶标之间的相互作用。为了研究STAT蛋白与抑制剂之间的相互作用,我们在本文中开发了STAT CETSA并评估了已知的STAT3抑制剂在生物学环境中与STAT蛋白结合的能力。虽然检测到STAT蛋白与肽STAT抑制剂之间有强力结合,但小分子抑制剂引起可变应答,其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。为了研究STAT蛋白与抑制剂之间的相互作用,我们在本文中开发了STAT CETSA并评估了已知的STAT3抑制剂在生物学环境中与STAT蛋白结合的能力。虽然检测到STAT蛋白与肽STAT抑制剂之间有强力结合,但小分子抑制剂引起可变应答,其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。为了研究STAT蛋白与抑制剂之间的相互作用,我们在本文中开发了STAT CETSA,并评估了已知的STAT3抑制剂在生物学环境中与STAT蛋白结合的能力。虽然检测到STAT蛋白与肽STAT抑制剂之间有强力结合,但小分子抑制剂引起可变应答,其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议的STAT抑制剂的有价值的工具。
更新日期:2020-07-17
中文翻译:

使用生化和细胞热位移分析验证信号转导和转录激活剂(STAT)蛋白抑制剂的相互作用。
信号转导子和转录激活子(STAT)蛋白具有重要的生物学功能。然而,STAT信号转导的失调是炎性疾病和癌症的发作和发展的驱动力。尽管它们的生物学作用表明STAT蛋白将成为开发治疗剂的重要靶标,但是众所周知,STAT蛋白很难用类药物小分子抑制,因为它们没有明显的抑制剂结合位点。尽管如此,已经提出了许多小分子STAT抑制剂,主要集中在抑制STAT3蛋白以产生新的癌症疗法上。过去,证明抑制剂与细胞中的靶标结合一直是一项非常艰巨的任务。随着现代目标交战技术的出现,例如细胞热位移分析(CETSA),可以相对轻松地检测实验化合物与其生物学靶标之间的相互作用。为了研究STAT蛋白与抑制剂之间的相互作用,我们在本文中开发了STAT CETSA并评估了已知的STAT3抑制剂在生物学环境中与STAT蛋白结合的能力。虽然检测到STAT蛋白与肽STAT抑制剂之间有强力结合,但小分子抑制剂引起可变应答,其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。为了研究STAT蛋白与抑制剂之间的相互作用,我们在本文中开发了STAT CETSA并评估了已知的STAT3抑制剂在生物学环境中与STAT蛋白结合的能力。虽然检测到STAT蛋白与肽STAT抑制剂之间有强力结合,但小分子抑制剂引起可变应答,其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。为了研究STAT蛋白与抑制剂之间的相互作用,我们在本文中开发了STAT CETSA,并评估了已知的STAT3抑制剂在生物学环境中与STAT蛋白结合的能力。虽然检测到STAT蛋白与肽STAT抑制剂之间有强力结合,但小分子抑制剂引起可变应答,其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议STAT抑制剂的有价值的工具。其中大多数未能稳定细胞和细胞裂解物中的STAT3蛋白。所描述的STAT热稳定性测定代表了评估拟议的STAT抑制剂的有价值的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号