Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-05-16 , DOI: 10.1016/j.csbj.2020.05.012 Wresti L Anggayasti 1 , Kenta Ogino 2 , Eiji Yamamoto 3 , Erik Helmerhorst 4 , Kenji Yasuoka 2 , Ricardo L Mancera 4
|
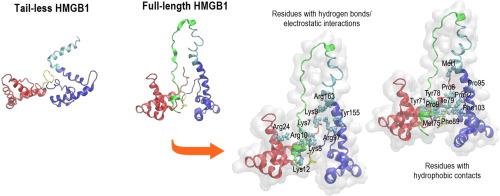
High mobility group box 1 (HMGB1) is a damage-associated molecular pattern (DAMP) molecule that triggers the progression of several pro-inflammatory diseases such as diabetes, Alzheimer’s disease and cancer, by inducing signals upon interaction with the receptors such as the receptor for advanced glycation end-products (RAGE) and toll-like receptors (TLRs). The acidic C-terminal tail of HMGB1 is an intrinsically disordered region of the protein which is known to determine the interaction of HMGB1 to DNA and histones. This study characterizes its structural properties using a combination of circular dichroism (CD) and molecular dynamics (MD) simulations. The full-length and tail-less forms of HMGB1 were compared to rationalise the role of the acidic tail in maintaining the stability of the entire structure of HMGB1 in atomistic detail. Consistent with experimental data, the acidic tail was predicted to adopt an extended conformation that allows it to make a range of hydrogen-bonding and electrostatic interactions with the box-like domains that stabilize the overall structure of HMGB1. Absence of the acidic tail was predicted to increase structural fluctuations of all amino acids, leading to changes in secondary structure from α-helical to more hydrophilic turns along with increased exposure of multiple amino acids to the surrounding solvent. These structural changes reveal the intrinsic conformational dynamics of HMGB1 that are likely to affect the accessibility of its receptors.
中文翻译:

HMGB1 的酸性尾调节其二级结构和构象灵活性:圆二色性和分子动力学模拟研究。
高迁移率族蛋白 1 (HMGB1) 是一种损伤相关分子模式 (DAMP) 分子,通过与受体(如受体)相互作用诱导信号,触发多种促炎性疾病(如糖尿病、阿尔茨海默病和癌症)的进展用于晚期糖基化终末产物 (RAGE) 和 Toll 样受体 (TLR)。HMGB1 的酸性 C 末端尾部是该蛋白质本质上无序的区域,已知该区域决定 HMGB1 与 DNA 和组蛋白的相互作用。本研究结合圆二色性 (CD) 和分子动力学 (MD) 模拟来表征其结构特性。对 HMGB1 的全长和无尾形式进行了比较,以合理化酸性尾在维持 HMGB1 整个结构的原子细节稳定性方面的作用。与实验数据一致,预计酸性尾部将采用扩展构象,使其能够与盒状结构域形成一系列氢键和静电相互作用,从而稳定 HMGB1 的整体结构。预计酸性尾部的缺失会增加所有氨基酸的结构波动,导致二级结构从 α 螺旋变为更亲水的转角,同时增加多种氨基酸与周围溶剂的接触。这些结构变化揭示了 HMGB1 的内在构象动力学,可能会影响其受体的可及性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号