Cell Stem Cell ( IF 19.8 ) Pub Date : 2020-05-15 , DOI: 10.1016/j.stem.2020.04.010 Rashim Pal Singh 1 , Danny V Jeyaraju 1 , Veronique Voisin 2 , Rose Hurren 1 , Changjiang Xu 2 , James R Hawley 1 , Samir H Barghout 1 , Dilshad H Khan 1 , Marcela Gronda 1 , Xiaoming Wang 1 , Yulia Jitkova 1 , David Sharon 1 , Sanduni Liyanagae 1 , Neil MacLean 1 , Ayesh K Seneviratene 1 , Sara Mirali 1 , Adina Borenstein 1 , Geethu E Thomas 1 , Joelle Soriano 1 , Elias Orouji 1 , Mark D Minden 1 , Andrea Arruda 1 , Steven M Chan 1 , Gary D Bader 2 , Mathieu Lupien 1 , Aaron D Schimmer 1
|
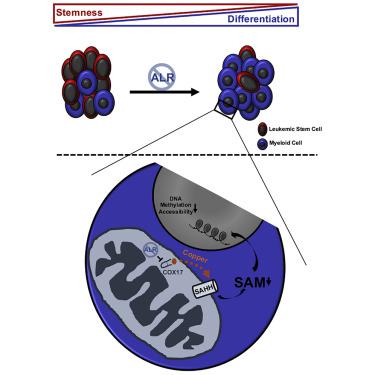
Leukemic stem cells (LSCs) rely on oxidative metabolism and are differentially sensitive to targeting mitochondrial pathways, which spares normal hematopoietic cells. A subset of mitochondrial proteins is folded in the intermembrane space via the mitochondrial intermembrane assembly (MIA) pathway. We found increased mRNA expression of MIA pathway substrates in acute myeloid leukemia (AML) stem cells. Therefore, we evaluated the effects of inhibiting this pathway in AML. Genetic and chemical inhibition of ALR reduces AML growth and viability, disrupts LSC self-renewal, and induces their differentiation. ALR inhibition preferentially decreases its substrate COX17, a mitochondrial copper chaperone, and knockdown of COX17 phenocopies ALR loss. Inhibiting ALR and COX17 increases mitochondrial copper levels which in turn inhibit S-adenosylhomocysteine hydrolase (SAHH) and lower levels of S-adenosylmethionine (SAM), DNA methylation, and chromatin accessibility to lower LSC viability. These results provide insight into mechanisms through which mitochondrial copper controls epigenetic status and viability of LSCs.
中文翻译:

破坏线粒体铜分布抑制白血病干细胞自我更新。
白血病干细胞(LSC)依赖于氧化代谢,并且对靶向线粒体途径具有不同的敏感性,而线粒体途径可保留正常的造血细胞。线粒体蛋白的一个子集通过线粒体膜间组装(MIA)途径在膜间空间折叠。我们发现在急性髓细胞白血病(AML)干细胞中MIA途径底物的mRNA表达增加。因此,我们评估了在AML中抑制该途径的作用。对ALR的遗传和化学抑制作用会降低AML的生长和生存能力,破坏LSC的自我更新并诱导其分化。ALR抑制优先降低其底物COX17,线粒体铜分子伴侣和降低COX17表型ALR损失。抑制ALR和COX17会增加线粒体铜水平,进而抑制S-腺苷同型半胱氨酸水解酶(SAHH)和较低水平的S-腺苷甲硫氨酸(SAM),DNA甲基化和染色质可及性,从而降低LSC活力。这些结果为线粒体铜控制LSC的表观遗传状态和生存力的机制提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号