Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-05-15 , DOI: 10.1016/j.apcata.2020.117607 Buchang Shi , Yunxin Liao , Zachary J. Callihan , Brad T. Shoopman , Mingsheng Luo
|
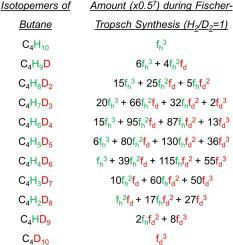
An inverse isotope effect and deuterium enrichments in hydrocarbons were observed in Fe catalyzed Fischer-Tropsch (FT) reactions. These results are explained by an Alkylidene mechanism by which the monomer of the polymerization-like reaction is MCH and the growing chain is RCH = M. The formation of C
C bond is through the reaction of M
CH and RCH = M, producing a new growing chain RCH2CH = M. During the process of C
C bond formation, the sp2 carbon in RCH = M is changed to sp3 in newly formed growing chain RCH2CH = M, which results in an inverse isotope effect. The rate of reaction of RCD = M with M
CH/M≡CD is fast than that of RCH = M with M
CH/M≡CD. The calculations based on alkylidene mechanism showed that the deuterium is enriched in hydrocarbons and that the deuterium enrichment is a function of carbon number.
中文翻译:

铁催化费-托合成过程中碳-碳键的形成
在铁催化的费-托反应中观察到了同位素的反作用和氘在烃中的富集。这些结果可以通过亚烷基机理来解释,其中类似聚合反应的单体是M CH,增长链是RCH =M。C
C键的形成是通过M
CH和RCH = M的反应生成的。新生长链RCH 2 CH = M.期间C的过程
C键的形成,对SP 2碳在RCH = M改变为SP 3中新形成的生长链RCH 2 CH = M,其结果在逆同位素效应。RCD = M与M
CH /M≡CD的反应速率快于RCH = M与M的反应速率
CH /M≡CD。基于亚烷基机理的计算表明,氘富含碳氢化合物,并且氘的富集是碳数的函数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号