当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arsenic immobilization as crystalline scorodite by gas-diffusion electrocrystallization
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-05-15 , DOI: 10.1039/d0re00054j Guillermo Pozo 1, 2, 3, 4, 5 , Diane van Houtven 1, 2, 3, 4, 5 , Jan Fransaer 5, 6, 7, 8, 9 , Xochitl Dominguez-Benetton 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-05-15 , DOI: 10.1039/d0re00054j Guillermo Pozo 1, 2, 3, 4, 5 , Diane van Houtven 1, 2, 3, 4, 5 , Jan Fransaer 5, 6, 7, 8, 9 , Xochitl Dominguez-Benetton 1, 2, 3, 4, 5
Affiliation
|
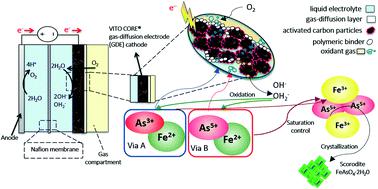
The safe immobilization of arsenic present in liquids is a key environmental challenge due to the inherent toxicity of arsenic. This immobilization is mostly restricted by the application of chemicals and several stages of oxidation and precipitation. Although the formation of bioscorodite is a greener alternative, it is intensive in the use of energy for aeration, and it is costly due to nutrient addition. The electrochemically-driven crystallization of arsenic into scorodite is proposed here to overcome these limitations. We disclose gas-diffusion electrocrystallization (GDEx) for the immobilization of arsenic into highly crystalline scorodite (FeAsO4·2H2O) by the in situ production of oxidizing substances (i.e., H2O2) on gas-diffusion electrodes. GDEx yielded an exceptional arsenic immobilization efficiency of up to 70% without the use of any primary minerals or seed crystals. At 70 °C and using As3+ as the precursor, polydisperse micrometric scorodite particles were obtained (from fine particles of <1 μm to large particles of ∼5 μm). In contrast, fine micrometric particles of <1 μm were achieved using As5+ as the precursor. Using one-pot and one-step GDEx enabled the synthesis of scorodite that was 14 times less soluble than required for stable scorodite disposal. Current chemical oxidation–precipitation processes use two separate reactors, including the oxidation of As3+ to As5+, and then the precipitation of the As5+ with Fe3+ to generate scorodite at a temperature higher than 90 °C. In contrast, the new GDEx approach combines both reactors into one to produce crystalline scorodite at 50 °C, hence reducing energy requirements and chemical footprint.
中文翻译:

气体扩散电结晶法将砷固定为结晶臭葱石
由于砷固有的毒性,液体中砷的安全固定是一项关键的环境挑战。这种固定化主要受化学品的应用以及氧化和沉淀的多个阶段的限制。尽管生物臭葱石的形成是一种更绿色的替代方法,但它在用于充气的能源方面消耗很大,并且由于添加了营养物而成本很高。为了克服这些局限性,这里提出了电化学驱动的砷化成臭葱石的结晶。我们公开了通过原位产生氧化性物质(即H 2)将砷固定化为高结晶臭葱石(FeAsO 4 ·2H 2 O)的气体扩散电结晶(GDEx )O 2)在气体扩散电极上。GDEx在不使用任何主要矿物质或种晶的情况下,砷的固定效率高达70%。在70°C且使用As 3+作为前驱体,获得了多分散微米级臭葱石颗粒(从<1μm的细颗粒到〜5μm的大颗粒)。相比之下,使用As 5+作为前体可实现<1μm的细微颗粒。使用一锅一步GDEx可以合成臭葱石,其溶解度比稳定臭葱石处理所需的溶解度低14倍。当前的化学氧化沉淀过程使用两个单独的反应器,包括将As 3+氧化为As 5+,然后沉淀As5+与Fe 3+在高于90°C的温度下生成臭葱石。相比之下,新的GDEx方法将两个反应器组合在一起,以在50°C的温度下生产结晶臭葱石,从而降低了能源需求和化学足迹。
更新日期:2020-05-15
中文翻译:

气体扩散电结晶法将砷固定为结晶臭葱石
由于砷固有的毒性,液体中砷的安全固定是一项关键的环境挑战。这种固定化主要受化学品的应用以及氧化和沉淀的多个阶段的限制。尽管生物臭葱石的形成是一种更绿色的替代方法,但它在用于充气的能源方面消耗很大,并且由于添加了营养物而成本很高。为了克服这些局限性,这里提出了电化学驱动的砷化成臭葱石的结晶。我们公开了通过原位产生氧化性物质(即H 2)将砷固定化为高结晶臭葱石(FeAsO 4 ·2H 2 O)的气体扩散电结晶(GDEx )O 2)在气体扩散电极上。GDEx在不使用任何主要矿物质或种晶的情况下,砷的固定效率高达70%。在70°C且使用As 3+作为前驱体,获得了多分散微米级臭葱石颗粒(从<1μm的细颗粒到〜5μm的大颗粒)。相比之下,使用As 5+作为前体可实现<1μm的细微颗粒。使用一锅一步GDEx可以合成臭葱石,其溶解度比稳定臭葱石处理所需的溶解度低14倍。当前的化学氧化沉淀过程使用两个单独的反应器,包括将As 3+氧化为As 5+,然后沉淀As5+与Fe 3+在高于90°C的温度下生成臭葱石。相比之下,新的GDEx方法将两个反应器组合在一起,以在50°C的温度下生产结晶臭葱石,从而降低了能源需求和化学足迹。











































 京公网安备 11010802027423号
京公网安备 11010802027423号