当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and anti-HBV activity of carbocyclic nucleoside hybrids with salient features of entecavir and aristeromycin
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-05-15 , DOI: 10.1039/d0md00059k Ramakrishnamraju Samunuri 1, 2, 3, 4, 5 , Masaaki Toyama 6, 7, 8, 9, 10 , Renuka Sivasankar Pallaka 4, 5, 11, 12 , Seshubabu Neeladri 4, 5, 11, 12 , Ashok Kumar Jha 4, 5, 11, 12 , Masanori Baba 6, 7, 8, 9, 10 , Chandralata Bal 1, 2, 3, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-05-15 , DOI: 10.1039/d0md00059k Ramakrishnamraju Samunuri 1, 2, 3, 4, 5 , Masaaki Toyama 6, 7, 8, 9, 10 , Renuka Sivasankar Pallaka 4, 5, 11, 12 , Seshubabu Neeladri 4, 5, 11, 12 , Ashok Kumar Jha 4, 5, 11, 12 , Masanori Baba 6, 7, 8, 9, 10 , Chandralata Bal 1, 2, 3, 4
Affiliation
|
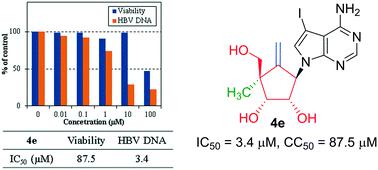
Modified carbocyclic nucleosides (4a–g) constituting 7-deazapurine, 4′-methyl, exocyclic double bond and 2′,3′-hydroxy were synthesized. NOE and X-ray studies of 4c confirmed the α-configuration of 4′-methyl. The anti-HBV assay demonstrated 4e (IC50 = 3.4 μM) without notable cytotoxicity (CC50 = 87.5 μM) as a promising lead for future exploration.
中文翻译:

具有恩替卡韦和阿霉素的突出特征的碳环核苷杂种的合成及抗HBV活性
合成了构成7-脱氮嘌呤,4'-甲基,环外双键和2',3'-羟基的修饰碳环核苷(4a-g)。4c的NOE和X射线研究证实了4'-甲基的α-构型。抗HBV检测证明4e(IC 50 = 3.4μM)没有明显的细胞毒性(CC 50 = 87.5μM),是未来探索的有希望的线索。
更新日期:2020-05-15
中文翻译:

具有恩替卡韦和阿霉素的突出特征的碳环核苷杂种的合成及抗HBV活性
合成了构成7-脱氮嘌呤,4'-甲基,环外双键和2',3'-羟基的修饰碳环核苷(4a-g)。4c的NOE和X射线研究证实了4'-甲基的α-构型。抗HBV检测证明4e(IC 50 = 3.4μM)没有明显的细胞毒性(CC 50 = 87.5μM),是未来探索的有希望的线索。









































 京公网安备 11010802027423号
京公网安备 11010802027423号