当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthesis of α-Substituted β-Ketoesters via the Tandem Knoevenagel Condensation–Reduction Reaction Using a Single Enzyme
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-15 , DOI: 10.1021/acssuschemeng.0c00938 Xiaolong Liu 1 , Xiangjie Li 1 , Zhelun Wang 1 , Jinlong Zhou 1 , Xinjiong Fan 1, 2 , Yao Fu 1, 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-05-15 , DOI: 10.1021/acssuschemeng.0c00938 Xiaolong Liu 1 , Xiangjie Li 1 , Zhelun Wang 1 , Jinlong Zhou 1 , Xinjiong Fan 1, 2 , Yao Fu 1, 3
Affiliation
|
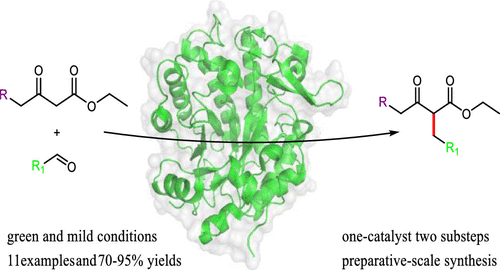
Saturated α-substituted β-ketoesters are important building blocks in the synthesis of pharmaceuticals and agrochemicals. Herein, we report a one-pot biosynthesis of α-substituted β-ketoesters via Knoevenagel condensation and reduction of the obtained unsaturated alkenes in situ, catalyzed by a single ene-reductase (NerA). A series of inexpensive and readily available aldehydes and 1,3-diketones were condensed and reduced by NerA in aqueous solutions at room temperature. We also noted that low loadings (3 mg/mL) of NerA were sufficient to facilitate the cascade process; both E and Z isomeric intermediates could be reduced effectively, and the overall yield was improved up to 95%. Meanwhile, the method could be applied to a gram preparative-scale synthesis of pharmaceutical intermediates. This process conformed to the concepts of green chemistry and showed advantages for the synthesis of high value saturated α-substituted β-ketoesters.
中文翻译:

通过单酶串联Knoevenagel缩合-还原反应生物合成α-取代的β-酮酸酯
饱和的α-取代的β-酮酸酯是药物和农用化学品合成中的重要组成部分。在这里,我们报道了通过单烯键还原酶(NerA)催化的Knoevenagel缩合和原位还原获得的不饱和烯烃一锅法合成α-取代的β-酮酸酯。在室温下,NerA在水溶液中将一系列廉价且易于获得的醛和1,3-二酮缩合并还原。我们还注意到,NerA的低负载量(3 mg / mL)足以促进级联过程。既Ë和ž异构中间体可以有效减少,总收率提高到95%。同时,该方法可应用于克制备规模的医药中间体的合成。该方法符合绿色化学的概念,并显示出合成高价值的饱和α-取代的β-酮酸酯的优势。
更新日期:2020-05-15
中文翻译:

通过单酶串联Knoevenagel缩合-还原反应生物合成α-取代的β-酮酸酯
饱和的α-取代的β-酮酸酯是药物和农用化学品合成中的重要组成部分。在这里,我们报道了通过单烯键还原酶(NerA)催化的Knoevenagel缩合和原位还原获得的不饱和烯烃一锅法合成α-取代的β-酮酸酯。在室温下,NerA在水溶液中将一系列廉价且易于获得的醛和1,3-二酮缩合并还原。我们还注意到,NerA的低负载量(3 mg / mL)足以促进级联过程。既Ë和ž异构中间体可以有效减少,总收率提高到95%。同时,该方法可应用于克制备规模的医药中间体的合成。该方法符合绿色化学的概念,并显示出合成高价值的饱和α-取代的β-酮酸酯的优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号